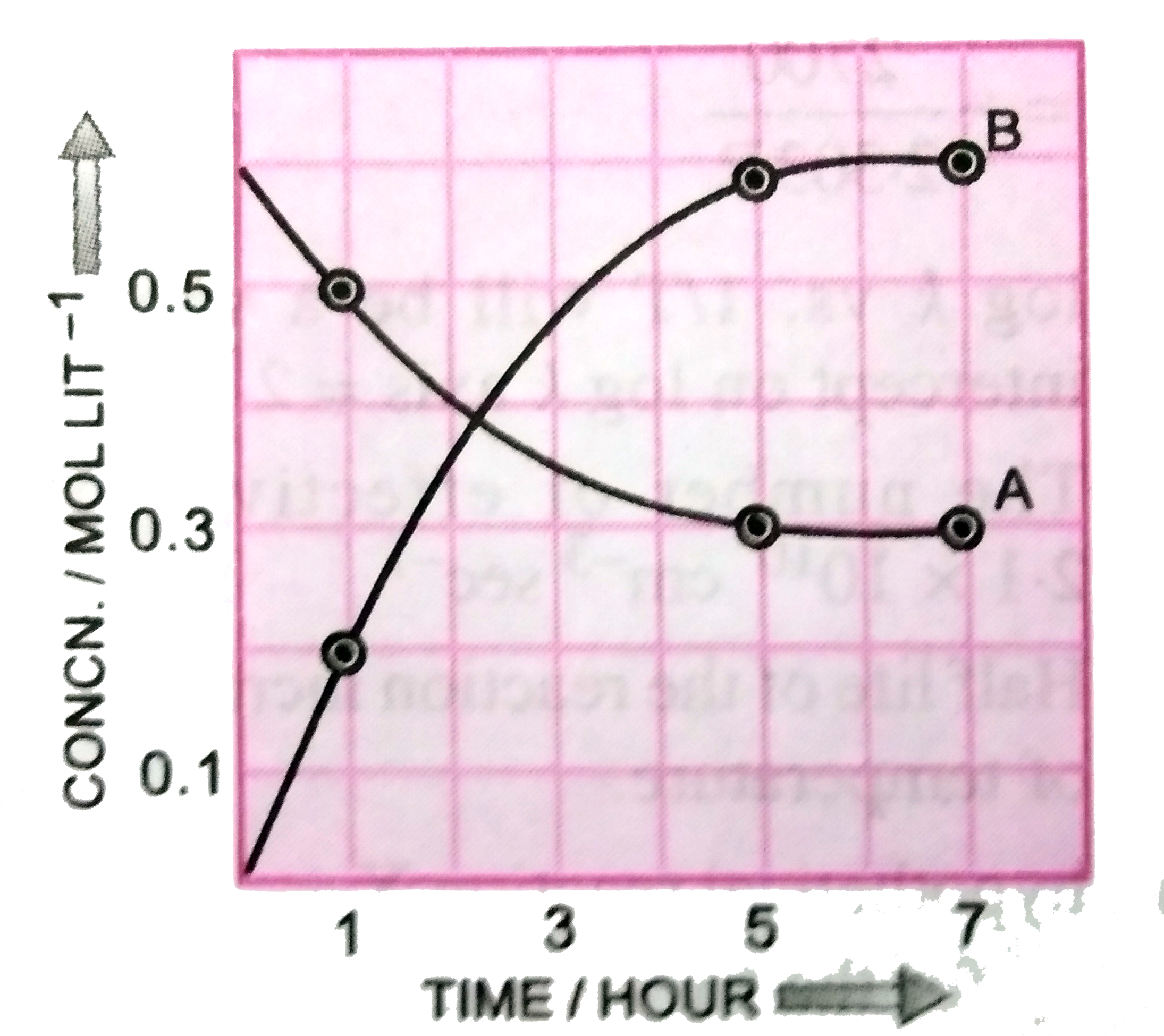
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
PRADEEP|Exercise COMPETITION FOCUS (IV. Matching Type Questions)|1 VideosCHEMICAL KINETICS
PRADEEP|Exercise COMPETITION FOCUS (JEE(Main and Advanced)/Medical Entrance) (IV. Matching Type Questions)|2 VideosCHEMICAL KINETICS
PRADEEP|Exercise COMPETITION FOCUS (JEE(Main and Advanced)/Medical Entrance) (I. Multiple Choice Questions) With one or more than one correct answers|7 VideosBIOMOLECULES
PRADEEP|Exercise IMPORTANT QUESTIONS (FOR BOARD EXAMINATION)|25 VideosCHEMISTRY IN EVERYDAY LIFE
PRADEEP|Exercise IMPORTANT QUESTION FOR BOARD EXAMINATION|30 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-CHEMICAL KINETICS-COMPETITION FOCUS (JEE(Main and Advanced)/Medical Entrance) (I. Multiple Choice Questions) (III. Multiple Choice Questions) Based on Comprehension
- Arrhenius studied the effect of temperature on the rate of a reaction ...
Text Solution
|
- Arrhenius studied the effect of temperature on the rate of a reaction ...
Text Solution
|
- Arrhenius studied the effect of temperature on the rate of a reaction ...
Text Solution
|
- Arrhenius studied the effect of temperature on the rate of a reaction ...
Text Solution
|
- Arrhenius studied the effect of temperature on the rate of a reaction ...
Text Solution
|
- The progress of the reaction A iff nB with time is represented in the ...
Text Solution
|
- The progress of the reaction A iff nB with time is represented in the ...
Text Solution
|
- The progress of the reaction A iff nB with time is represented in the ...
Text Solution
|