Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PRADEEP-CHEMICAL KINETICS-ADVANCED PROBLEMS FOR COMPETITIONS
- A flask contains a mixture of two compounds AB and XY. Both of these d...
Text Solution
|
- For a reaction A to Products, starting with initial concentrations of ...
Text Solution
|
- Two substances A and B are present such that [A(0)]=4[B(0] and half-l...
Text Solution
|
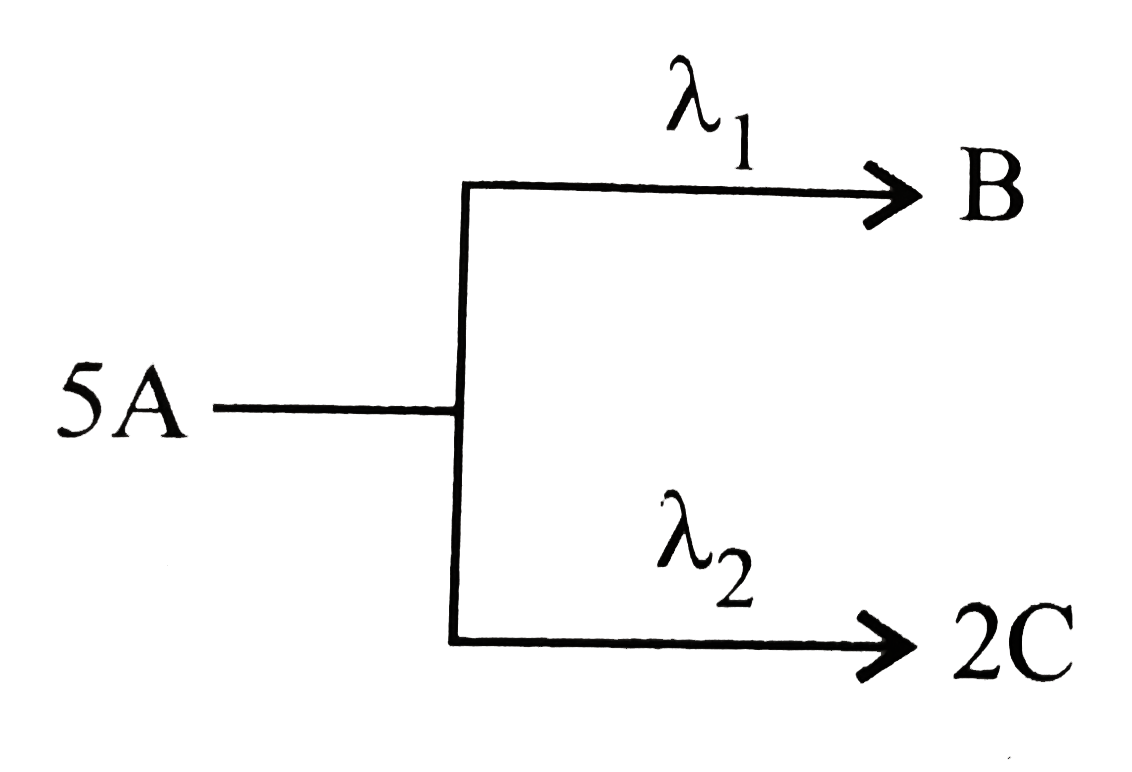
- A follow parallel path of first-order reactions giving B and C as If...
Text Solution
|
- Given that the enthalpy change of a reaction at 325 K is 0.12 kcal and...
Text Solution
|
- A(g)overset(Delta)rarrP(g)+Q(g)+R(g), follows first order kinetics wit...
Text Solution
|
- A drop of solution (volume 0.05 mL) contains 3 xx 10^(-6) "mole" H^(o+...
Text Solution
|
- The first order reaction: Sucrose rarr Glucose + Fructose takes plac...
Text Solution
|
- The rate of decomposition for methyl nitrite and ethyl nitrite can be ...
Text Solution
|
- The rate constant for the reaction COCl(2)(g)toCO(g)+Cl(2)(g) is given...
Text Solution
|
- A hydrogenation reaction is carried out at 500 K. If the same reaction...
Text Solution
|
- The energy change accompanying the equilibrium reaction A iff B is -33...
Text Solution
|
- Two reaction, (I)A rarr Products and (II) B rarr Products, follow firs...
Text Solution
|
- A certain reaction A+BtoP is of first order with respect to each react...
Text Solution
|