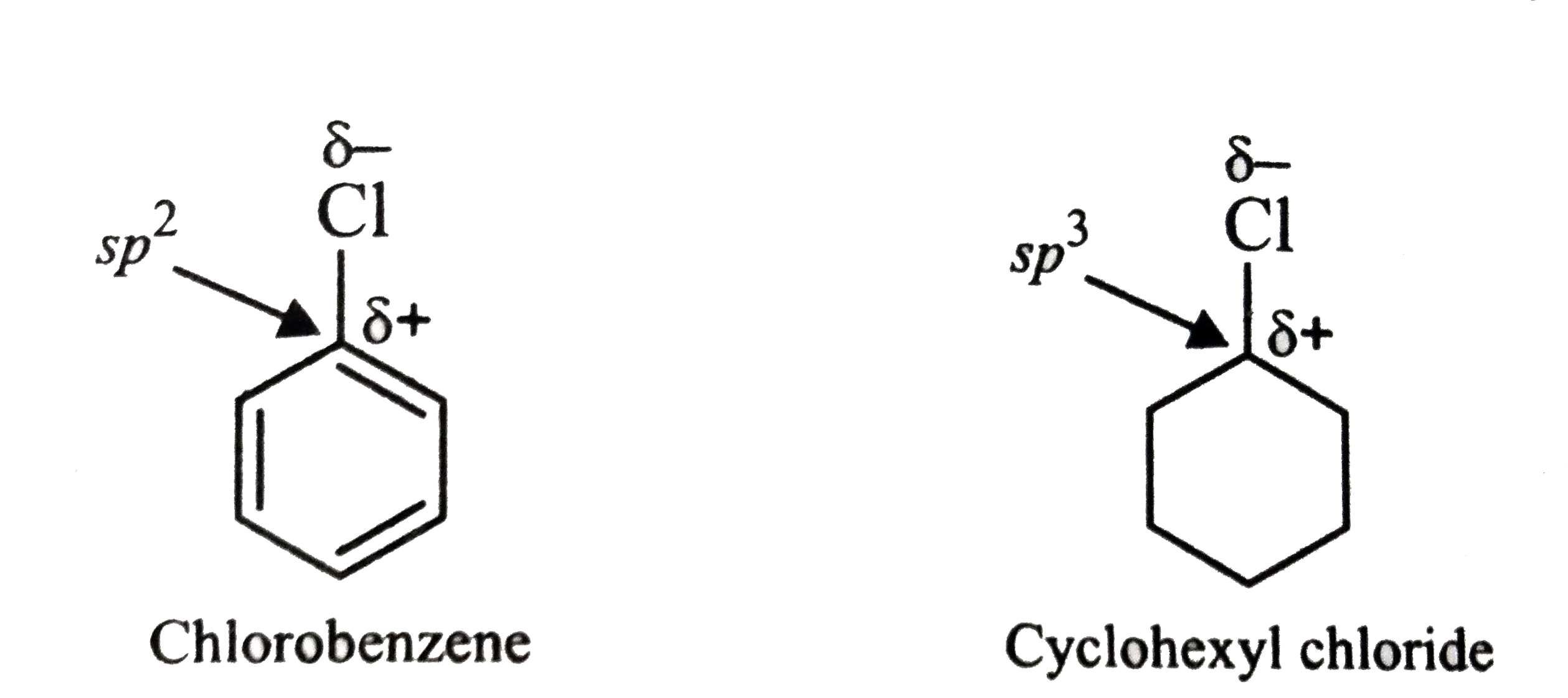
Text Solution
Verified by Experts
Topper's Solved these Questions
HALOALKANES AND HALOARENES
PRADEEP|Exercise ADDITIONAL QUESTIONS (SHORT ANSWER QUESTIONS)|47 VideosHALOALKANES AND HALOARENES
PRADEEP|Exercise ADDITIONAL QUESTIONS (LONG ANSWER QUESTIONS)|4 VideosHALOALKANES AND HALOARENES
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (LONG ANSWER QUESTIONS)|3 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
PRADEEP|Exercise Curiosity Questions|2 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|27 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-HALOALKANES AND HALOARENES-ADDITIONAL QUESTIONS (VERY SHORT ANSWER QUESTIONS)
- C(C(6)H(12)), and optically active dydrocarbon which on catalytic hydr...
Text Solution
|
- Which out of the two : 2-cyclopentenol or 3-cyclopentenol has chiral c...
Text Solution
|
- Why is the seperation of lanthanoids difficult ?
Text Solution
|
- Write a chemical reaction in which the iodide ion replaces the diazoni...
Text Solution
|
- What effect should the following resonance of vinyl chloride have on i...
Text Solution
|
- Why is vinyl chloride less reactive than ethyl chloride ?
Text Solution
|
- Which of the following is most reactive toward nucleophilic substituti...
Text Solution
|
- Give reasons: (i). C-Cl bond length in chlorobenzene is shorter than...
Text Solution
|
- The dipole moment of vinyl chloride is lower than that of methyl chlor...
Text Solution
|
- Grignard reagent of C(6)H(5)Cl can be prepared in THF but not in ether...
Text Solution
|
- Give reasons: (a) n-Butyl bromide has higher boiling point than t-bu...
Text Solution
|
- Arrange the following in the increasing order of ease of nucleo...
Text Solution
|
- Give one example (with equation) wurtz-fittig reaction.
Text Solution
|
- How will you convert: chlorobenzene to biphenyl?
Text Solution
|
- Arrange the following in increasing order of reactivity towards sulpho...
Text Solution
|
- How is DDT prepared?
Text Solution
|
- Chloroform is stored in dark coloured bottles. Explain in not more t...
Text Solution
|
- How will you test pure chloroform?
Text Solution
|
- A small amount of ethyl alcohol is usually added to chloroform bottles...
Text Solution
|
- Why iodoform show antiseptic properties ?
Text Solution
|