Text Solution
Verified by Experts
Topper's Solved these Questions
HALOALKANES AND HALOARENES
PRADEEP|Exercise HOTS PROBLEMS|1 VideosHALOALKANES AND HALOARENES
PRADEEP|Exercise VALUE BASED QUESTIONS WITH ANSWERS|4 VideosHALOALKANES AND HALOARENES
PRADEEP|Exercise ADDITIONAL QUESTIONS (LONG ANSWER QUESTIONS)|4 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
PRADEEP|Exercise Curiosity Questions|2 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|27 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-HALOALKANES AND HALOARENES-HIGHER ORDER THINKING SKILLS ((QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTION)
- Why alkyl halides are generally not prepared in the laboratory by free...
Text Solution
|
- Explain why chlorination of n-butane in presence of light at 298 K gi...
Text Solution
|
- Wurtz reaction fails in case of tert-alkyl halides. Explain.
Text Solution
|
- Explain the followinng in one or two sentences (i) Displacement of cya...
Text Solution
|
- Consider the following structure: Which of these structure is/are...
Text Solution
|
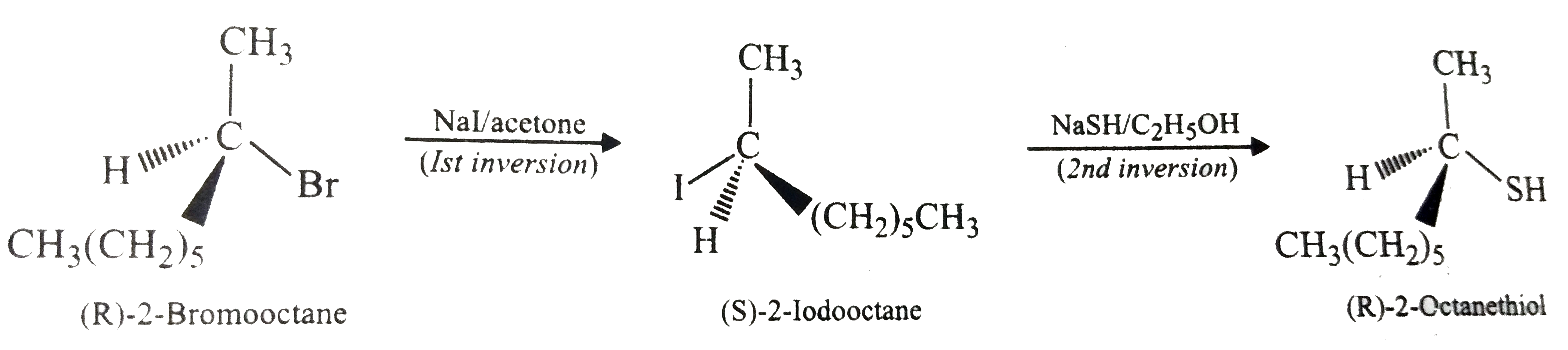
- (R)-2-Bromooctane reacts with NaSH to form (S)-2-octanethiol with inve...
Text Solution
|