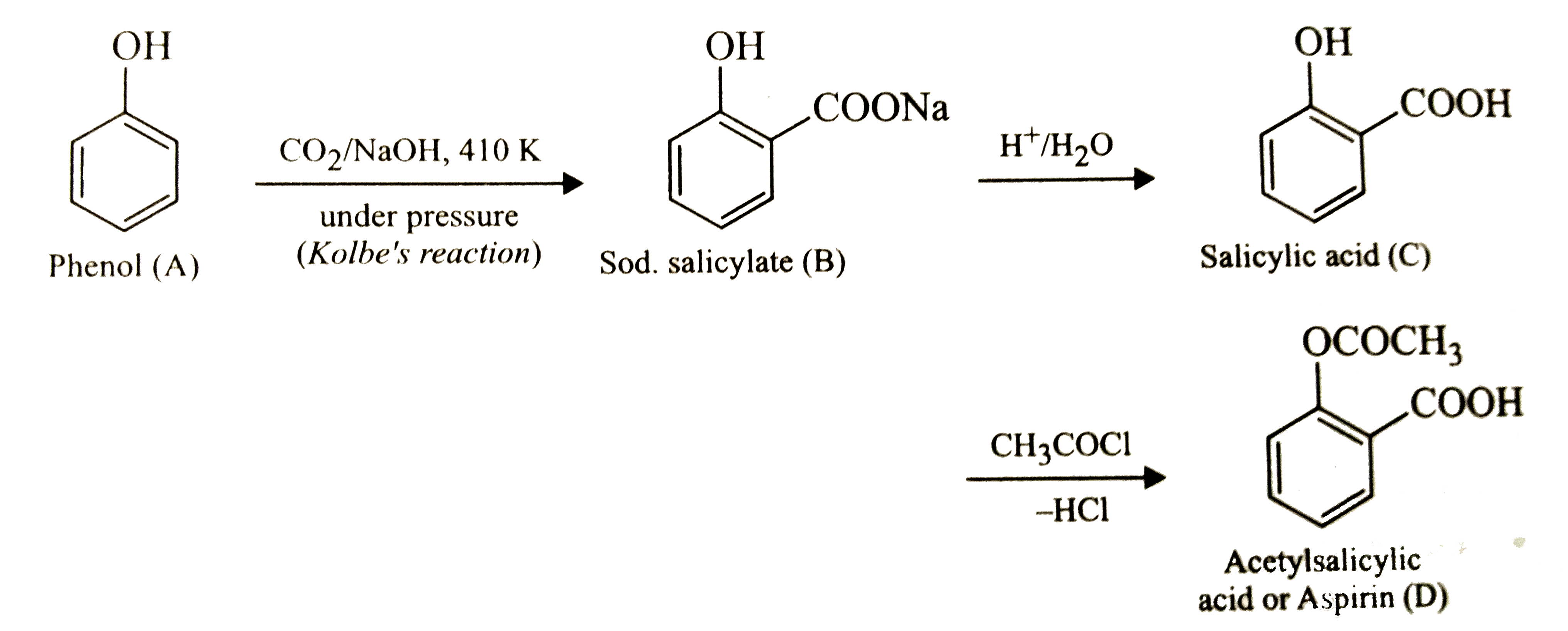
Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise VALUE BASED QUESTIONS WITH ANSWERS|4 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCES SPECIAL (MULTIPLE CHOICE QUESTIONS WITH ONE CORRECT ANSWER-I)|97 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise HIGHER ORDER THINKING SKILLS (QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTION)|5 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|29 Videos
Similar Questions
Explore conceptually related problems