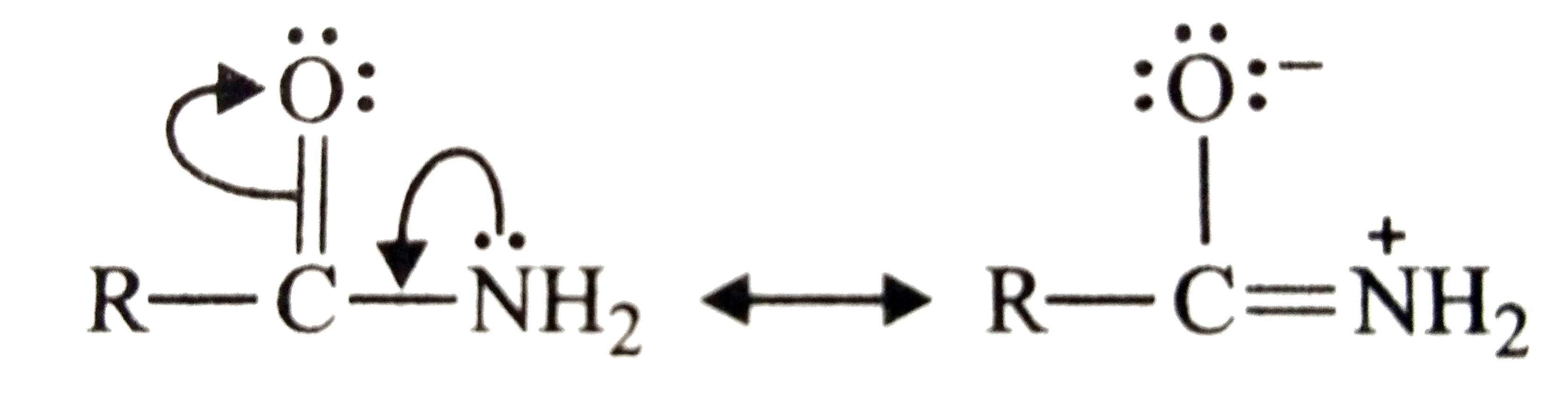Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
PRADEEP|Exercise HIGHER ORDER THINKING SKILLS (HOTS PROBLEMS)|2 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
PRADEEP|Exercise VALUE BASED QUESTIONS WITH ANSWERS|3 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
PRADEEP|Exercise ADDITIONAL QUESTIONS (LONG ANSWER QUESTIONS)|7 VideosHALOALKANES AND HALOARENES
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|22 VideosP-BLOCK ELEMENTS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|25 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ORGANIC COMPOUNDS CONTAINING NITROGEN-HIGHER ORDER THINKING SKILLS (HOTS QUESTIONS)
- How is 2,4-dinitrophenylhydrazine prepared from chlorobenzene?
Text Solution
|
- How will you convert toluene into sym-trinitrobenzene?
Text Solution
|
- tert-Butylamine cannot be prepared by action of ammonia on tert-butyl ...
Text Solution
|
- Why is an amide more acidic than amine?
Text Solution
|
- Which one is more acidic ? Explain.
Text Solution
|
- Arrange the following amines in order of decreasing basicity:
Text Solution
|
- Why does bromination of aniline, even under very mild conditions, give...
Text Solution
|