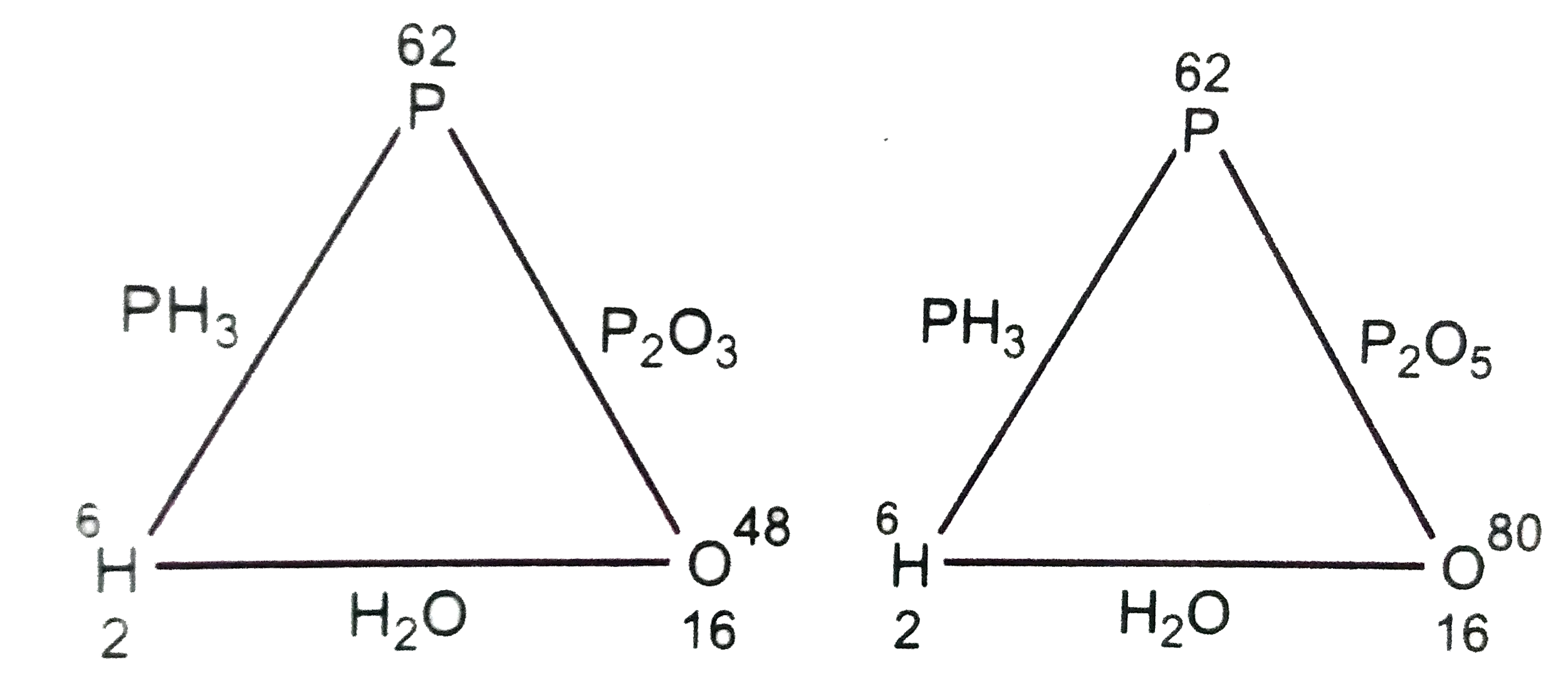
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOME BASIC CONCEPTS OF CHEMISTRY
PRADEEP|Exercise Competition (FOCUS) JEE (Main and Advanced)/Medical Entrance SPECIAL (II. Multiple Choice Question )|4 VideosSOME BASIC CONCEPTS OF CHEMISTRY
PRADEEP|Exercise Competition (FOCUS) JEE (Main and Advanced)/Medical Entrance SPECIAL (III. Multiple Choice Question )|6 VideosSOME BASIC CONCEPTS OF CHEMISTRY
PRADEEP|Exercise ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)|20 VideosSOLUTIONS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|31 VideosSURFACE CHEMISTRY
PRADEEP|Exercise IMPORTANT QUESTIONS (FOR BOARD EXAMINATION)|28 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-SOME BASIC CONCEPTS OF CHEMISTRY-Competition (FOCUS) JEE (Main and Advanced)/Medical Entrance SPECIAL (I. Multiple Choice Question )
- The number of significant figures in pi are
Text Solution
|
- Two oxides of a metal contain 36.4% and 53.4% of oxygen by mass respec...
Text Solution
|
- Which one of the following sets of compounds correctly illustrate the ...
Text Solution
|
- 116 mg of a compound on vaporisation in a victor Meyer's apparatus dis...
Text Solution
|
- An element X has the following isotopic composition : .^(200)X:90%,"...
Text Solution
|
- 1L of a gas is at a pressure of 10^(-6) mm of Hg at 25^(@)C. How many ...
Text Solution
|
- If 10^(21) molecules are removed from 200 mg of CO(2), the number of m...
Text Solution
|
- The weight of a molecule of the compoundC(60)H(122) is
Text Solution
|
- 10dm^(3) of N(2) gas and 10 dm^(3) of gas X at the same temperature co...
Text Solution
|
- How many moles of electrons weigh one kilogram?
Text Solution
|
- The total number of electrons in 18 mL of water ("density = 1 g "mL^(-...
Text Solution
|
- A gas mixture contains 50% helium and 50% methane by volume. What is t...
Text Solution
|
- If 1//6, in place of 1//12, mass of carbon atom is taken to be the rel...
Text Solution
|
- A person has as many notes as number of oxygen atoms in 24.8 g Na(2)S(...
Text Solution
|
- Number of mole of 1 m^(3) gas at NTP are:
Text Solution
|
- Volume occupied by one molecule of water (density = 1 g cm^(-3))
Text Solution
|
- The mass of 2.24xx10^(-3)m^(3) of a gas is 4.4 g at 273.15 K and 101.3...
Text Solution
|
- The total number of atoms of all elements present in mole of ammonium ...
Text Solution
|
- If 1 ml of water contains 20 drops. Then no. of molecules in a drop of...
Text Solution
|
- Which has the maximum number of molecules among the following ?
Text Solution
|