A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOME BASIC CONCEPTS OF CHEMISTRY
PRADEEP|Exercise Competition (FOCUS) JEE (Main and Advanced)/Medical Entrance SPECIAL (II. Multiple Choice Question )|4 VideosSOME BASIC CONCEPTS OF CHEMISTRY
PRADEEP|Exercise Competition (FOCUS) JEE (Main and Advanced)/Medical Entrance SPECIAL (III. Multiple Choice Question )|6 VideosSOME BASIC CONCEPTS OF CHEMISTRY
PRADEEP|Exercise ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)|20 VideosSOLUTIONS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|31 VideosSURFACE CHEMISTRY
PRADEEP|Exercise IMPORTANT QUESTIONS (FOR BOARD EXAMINATION)|28 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-SOME BASIC CONCEPTS OF CHEMISTRY-Competition (FOCUS) JEE (Main and Advanced)/Medical Entrance SPECIAL (I. Multiple Choice Question )
- For the formation of 3.65 g of hydrogen chloride gas, what volumes of ...
Text Solution
|
- The mass of carbon anode consumed (giving only carbon dioxide) in the ...
Text Solution
|
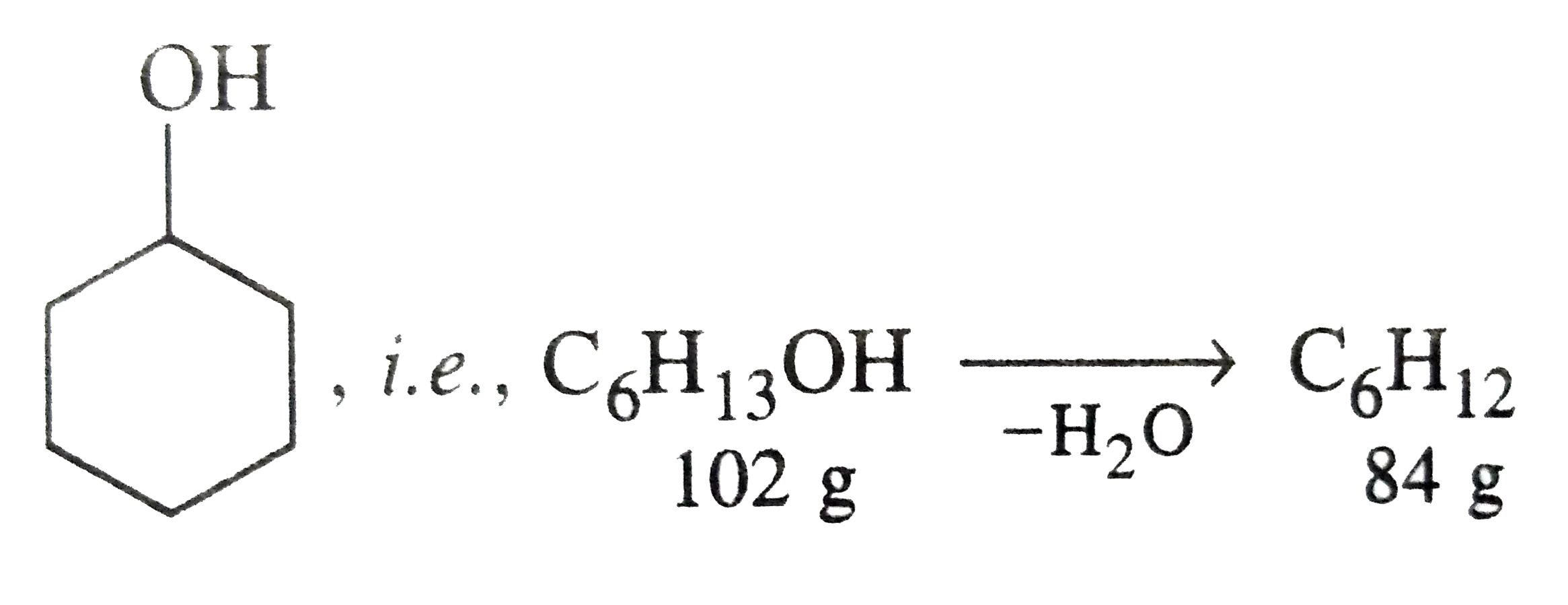
- The dehydration yield of cyclohexanol to cyclohexene is 75%. What woul...
Text Solution
|
- A mixture of CO(2) and CO is passed over red hot graphite when 1 mole...
Text Solution
|
- 3.28 g of a sample of pure copper when heated in presence of oxygen of...
Text Solution
|
- An ore contains 1.24% of the mineral argentite, Ag(2)S by mass. How ma...
Text Solution
|
- The decomposition of cetian mass of CaCO(3) gave 11.2dm^(3) of CO(2) g...
Text Solution
|
- 20.0 g of magnesium carbonate sample decomposes on heating to give car...
Text Solution
|
- A mixture of CaCl(2) and NaCl weighing 4.44 is treated with sodium car...
Text Solution
|
- A mixture of ethane and ethene occupies 41 L at atm and 500 K. The mix...
Text Solution
|
- Express of CO(2) is passed through 50 mL of 0.5 M calcium hydroxide so...
Text Solution
|
- In the reaction, 4NH(3)(g)+5O(2)(g) rarr 4NO(g)+6H(2)O(g), when 1 mole...
Text Solution
|
- 20 mL of methane is completely burnt using 50 mL of oxygen. The volume...
Text Solution
|
- The number of Cl^(-) ions in 100 mL of 0.001 M HCl solution is
Text Solution
|
- 50 mL solution of BaCl(2) (20.8% w//v) and 100 mL solution of H(2)SO(4...
Text Solution
|
- What is the mass of the precipitate formed when 50 mL of 16.9% solutio...
Text Solution
|
- How many moles of lead (II) chloride will be formed from a reaction be...
Text Solution
|
- 1 gram of carbonate (M(2)CO(3)) on treatment with excess HCl produces ...
Text Solution
|
- For reaction A+2BtoC. The amount of C formed by starting the reaction ...
Text Solution
|
- In an experiment, 4g of M(2)O(x) oxide was reduced to 2.8g of the met...
Text Solution
|