A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-KINETIC THEORY OF GASES-C.U.Q
- When heat is added to a system at constant temperature, which of the f...
Text Solution
|
- The first law of thermodynamics is based on the law of conservation of
Text Solution
|
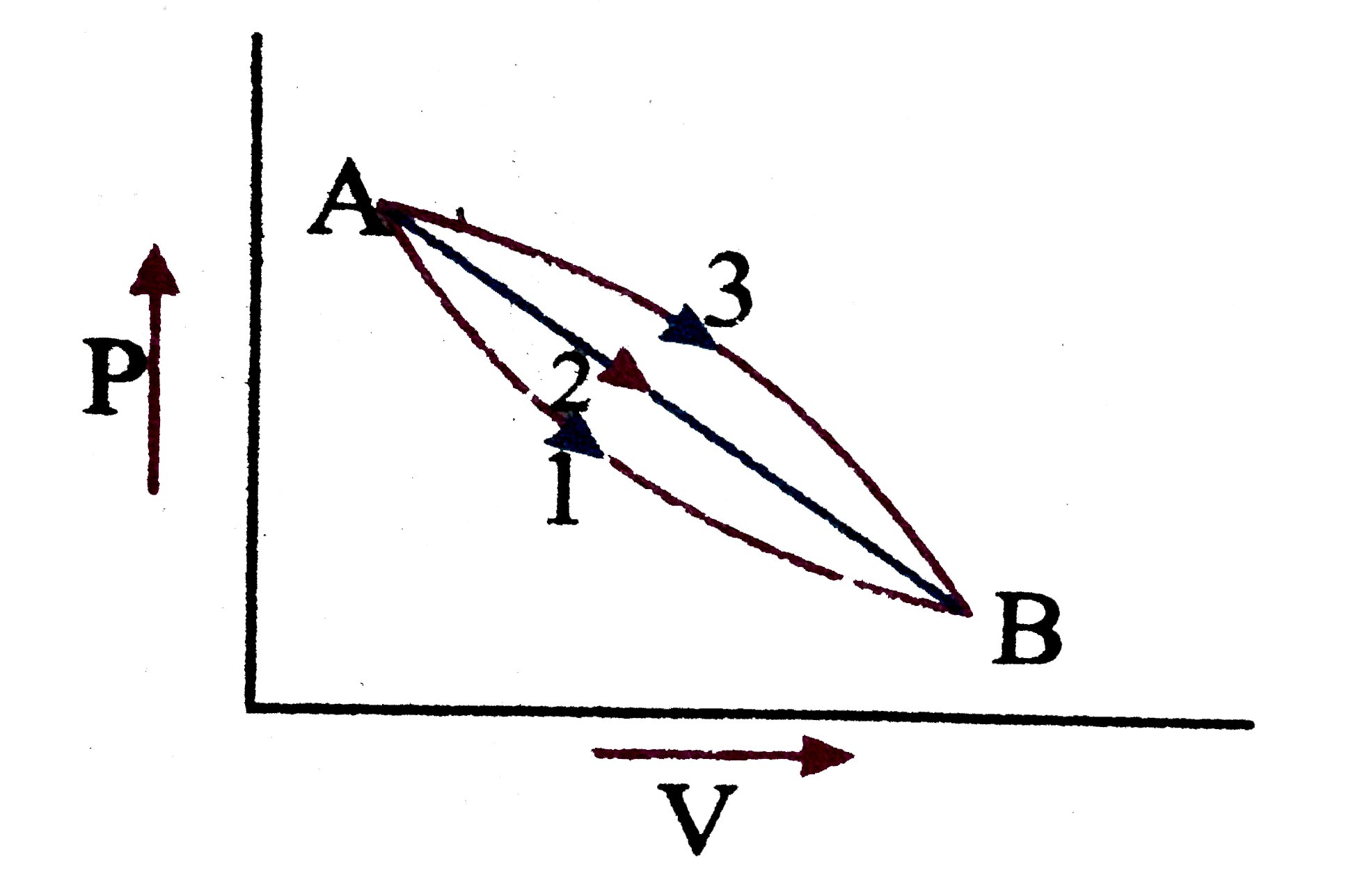
- A given mass of a gas expands from the state A to the state B by three...
Text Solution
|
- A given system undergoes a change in which the work done by the system...
Text Solution
|
- A closed vessel contains some gas at a given temperature and pressure....
Text Solution
|
- Unit mass of liquid of volume V(1) completely turns into a gas of volu...
Text Solution
|
- Find the ratio of (DeltaQ)/(DeltaU) and (DeltaQ)/(DeltaW) in an isobar...
Text Solution
|
- Find the ratio of (DeltaQ)/(DeltaU) and (DeltaQ)/(DeltaW) in an isobar...
Text Solution
|
- A gas is contained in a metallic cylinder fitted with a piston.The pis...
Text Solution
|
- The gases have two principal specific heats but solids and liquied hav...
Text Solution
|
- What is specific heat of gas in isothermal changes?
Text Solution
|
- At a given temperature, the specific heat of a gas at constant pressur...
Text Solution
|
- The ratio [C(p)//C(v)] of the specific heats at a constant pressure an...
Text Solution
|
- Which of the following formula is wrong?
Text Solution
|
- Two identical samples of gases are allowed to expand to the same final...
Text Solution
|
- Which of the following is true in the case of a reversible process?
Text Solution
|
- The ratio of the relative rise in pressure for adiabatic compression t...
Text Solution
|
- Ratio of isothermal elasticity of gas to the adiabatic elasticity is
Text Solution
|
- The conversion of water into ice is and
Text Solution
|
- For the Boyle's law to hold good, the necessary condition is
Text Solution
|