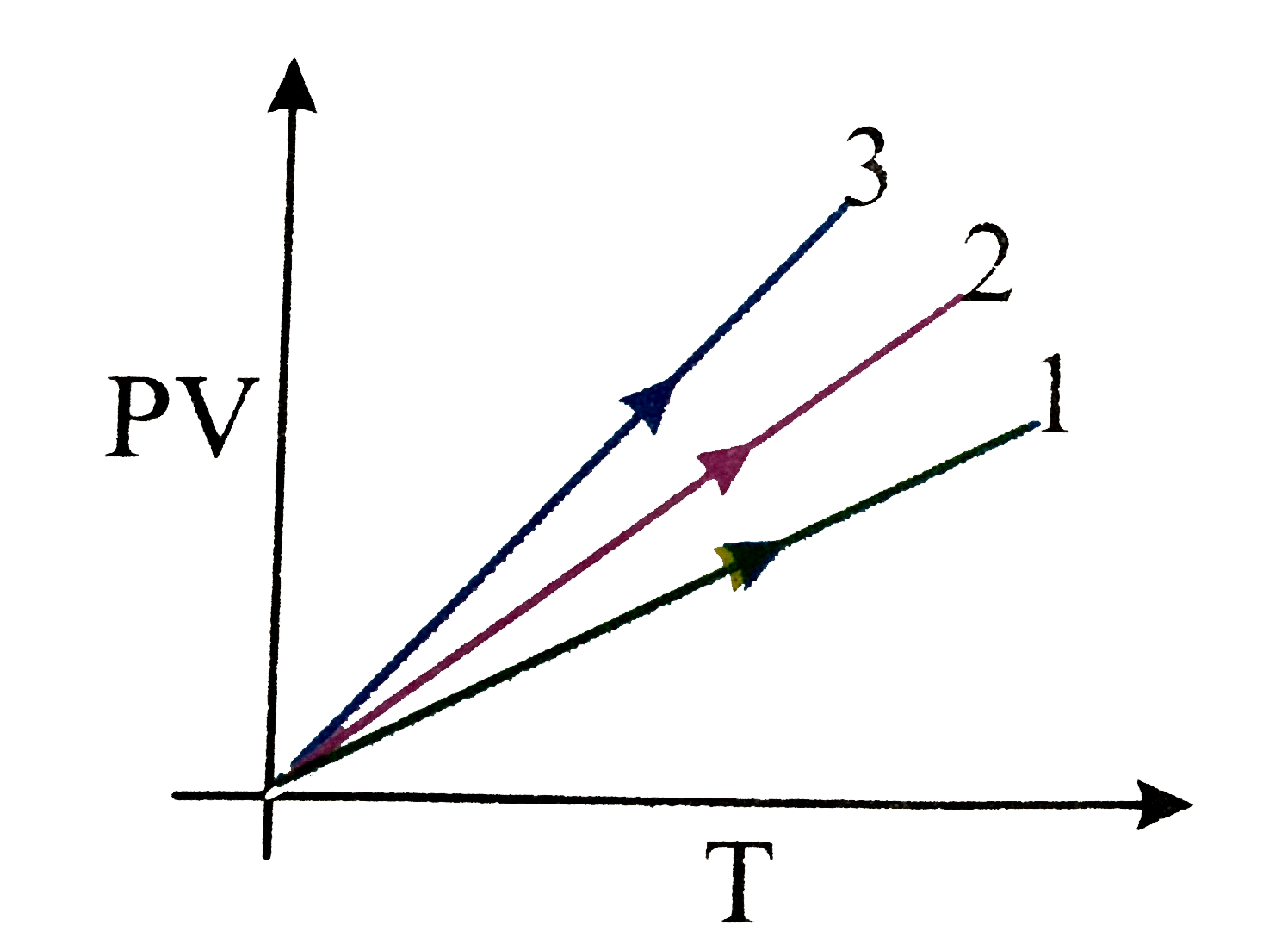A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-KINETIC THEORY OF GASES-C.U.Q
- Heat engine rejects some heat to the sink. This heat
Text Solution
|
- For an adiabatic change in a gas, if P,V,T denotes pressure, volume an...
Text Solution
|
- PV versus T graph of equal masses of H(2), He and CO(2) is shown in fi...
Text Solution
|
- If the ratio of specific heat of a gas of constant pressure to that at...
Text Solution
|
- Heat is added to an ideal gas and the gas expands. In such a process t...
Text Solution
|
- First law of thermodynamics states that
Text Solution
|
- Which of the following has maximum specific heat?
Text Solution
|
- The law obeyed by isothermal process is
Text Solution
|
- Which law defines entropy in thermodynamics
Text Solution
|
- For the conversion of liquid into a solid
Text Solution
|
- Among the following the irreversibel process is
Text Solution
|
- Which of the following processes are reversible ?
Text Solution
|
- Gas is taken through a cyclic process completely once. Change in the i...
Text Solution
|
- In following figs. Variation of volume by change of pressure is shown ...
Text Solution
|
- Which of the following is incorrect regarding the first law of thermod...
Text Solution
|
- The temperature of the system decreases in the process of
Text Solution
|
- The pressure p and volume V of an ideal gas both increase in a process...
Text Solution
|
- The heat capcity of material depends upon
Text Solution
|
- "Heat cannot by itself flow from a body at lower temperature to a body...
Text Solution
|
- For an isothermal process
Text Solution
|