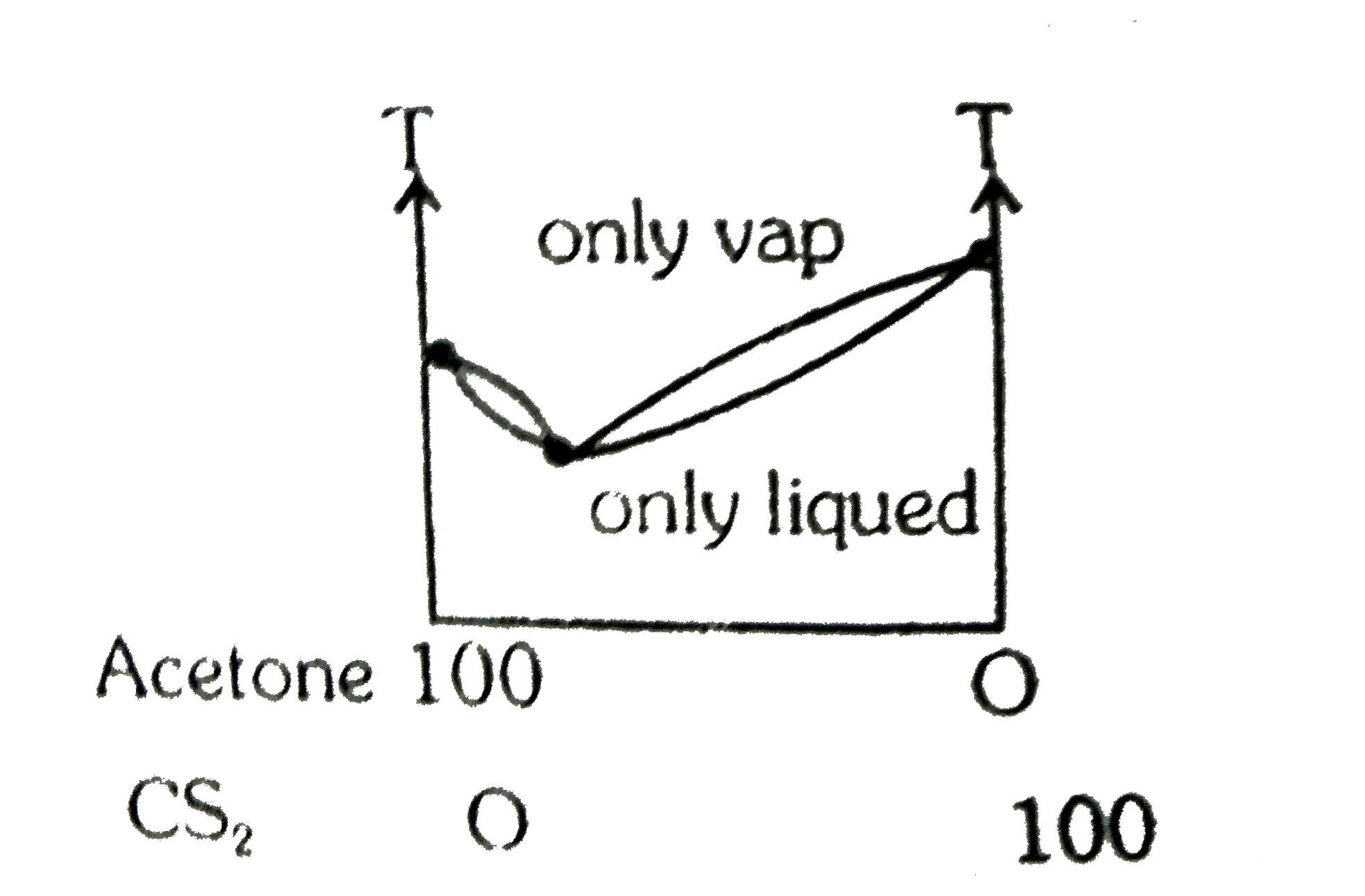
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-SOLUTIONS-EXERCISE -02
- For an ideal solution containing a nonvolatile solute, which of the fo...
Text Solution
|
- For a dilute solution containing a nonvolatile solute, the molar mass ...
Text Solution
|
- For a silute solution containing a nonvolatile solute, the molar mass ...
Text Solution
|
- An aqueous solution of acetone, CH(3)COCH(3) is 10.00% acetone by weig...
Text Solution
|
- The freezing poing of an aqueous solution of a non-electrolyte is -0.1...
Text Solution
|
- P(A)=(235y -125xy)mm of HgP(A) is partial pressure of A,x is mole frac...
Text Solution
|
- Of the following measurements the one most suitable for the determinat...
Text Solution
|
- The vapour pressure of pure benzeen at 50^(@)C is 268 torr. How many m...
Text Solution
|
- If P^(@) the vapour pressure of a pure solvent and P is the vapour pre...
Text Solution
|
- Dry air was passed successively through a solution of 5g of a solute i...
Text Solution
|
- The relative lowering of vapour pressure is equal to the ratio between...
Text Solution
|
- The vapour pressure of pure liquid solvent A is 0.80atm.When a non vol...
Text Solution
|
- Which of the following plots represents an ideal binary mixture?
Text Solution
|
- The lowering of vapour pressure in a saturated aq. Solution of salt AB...
Text Solution
|
- Which of the following represents correcty the changes in thermodynami...
Text Solution
|
- FeCI(3) on reaction with K(4)[Fe(CN)(6)] in aqueous solution gives blu...
Text Solution
|
- A liquid mixtue having composition corresponding to point z in the fig...
Text Solution
|
- The following graph represents variation of boiling point with composi...
Text Solution
|
- Which of the following is correct for a non-ideal solution of liquids ...
Text Solution
|
- Acetone and carbon disulphide form binary liquid solution showing pos...
Text Solution
|