A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-METALLURGY-EXERCISE-03
- {:(Column-I(Ore),Column-II("Created formula & properties")),((A)"Iron ...
Text Solution
|
- {:(Column-I("Metal"),column-II),((A)"Magnesite",(p)"Ore of magnesium")...
Text Solution
|
- {:(Column-I(Ore),Column-II),((A)"Iron",(p)"Carbon reduction method"),(...
Text Solution
|
- Statement-I : All the ores are mineral Statement-II : Most of the or...
Text Solution
|
- Statement-I : In the extraction of Ag the complex Na[Ag(CN)(2)] is rea...
Text Solution
|
- Statement-I : Thermite mixture Fe(2)O(3) +AI (powder) is used in the w...
Text Solution
|
- Statement-I : CuFeS(2) is concentrated by froath floatation method S...
Text Solution
|
- Statement-I : In the smelting of copper ore coke is added in the blas...
Text Solution
|
- Statement-I : Extraction of iron metal from iron oxide ore is carried ...
Text Solution
|
- Statement-I : Wolframite impurities are separated from cassiterite by ...
Text Solution
|
- Statement-I : Lead, tin the bismuth are purified by liquation method. ...
Text Solution
|
- Dow's process of extraction of Mg involves extraction of Mg from sea w...
Text Solution
|
- Dow's process of extraction of Mg involves extraction of Mg from sea w...
Text Solution
|
- Dow's process of extraction of Mg involves extraction of Mg from sea w...
Text Solution
|
- Dow's process of extraction of Mg involves extraction of Mg from sea w...
Text Solution
|
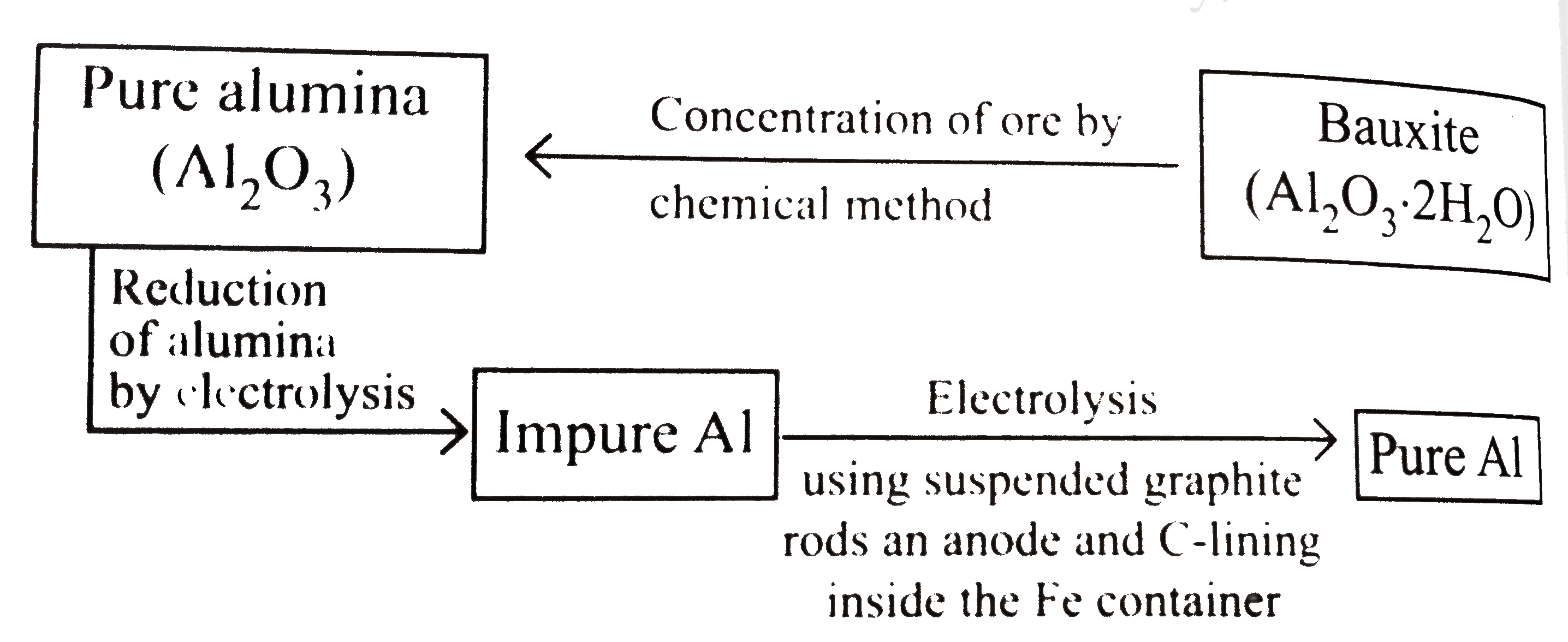
- Extraction of aluminium can be understood by : Electrolytric redu...
Text Solution
|
- Extraction of aluminium can be understood by : Electrolytric redu...
Text Solution
|
- Extraction of aluminium can be understood by : Electrolytric redu...
Text Solution
|
- Extraction of aluminium can be understood by : Electrolytric redu...
Text Solution
|
- Extraction of Aluminium can be understand by: electrolyte reducti...
Text Solution
|