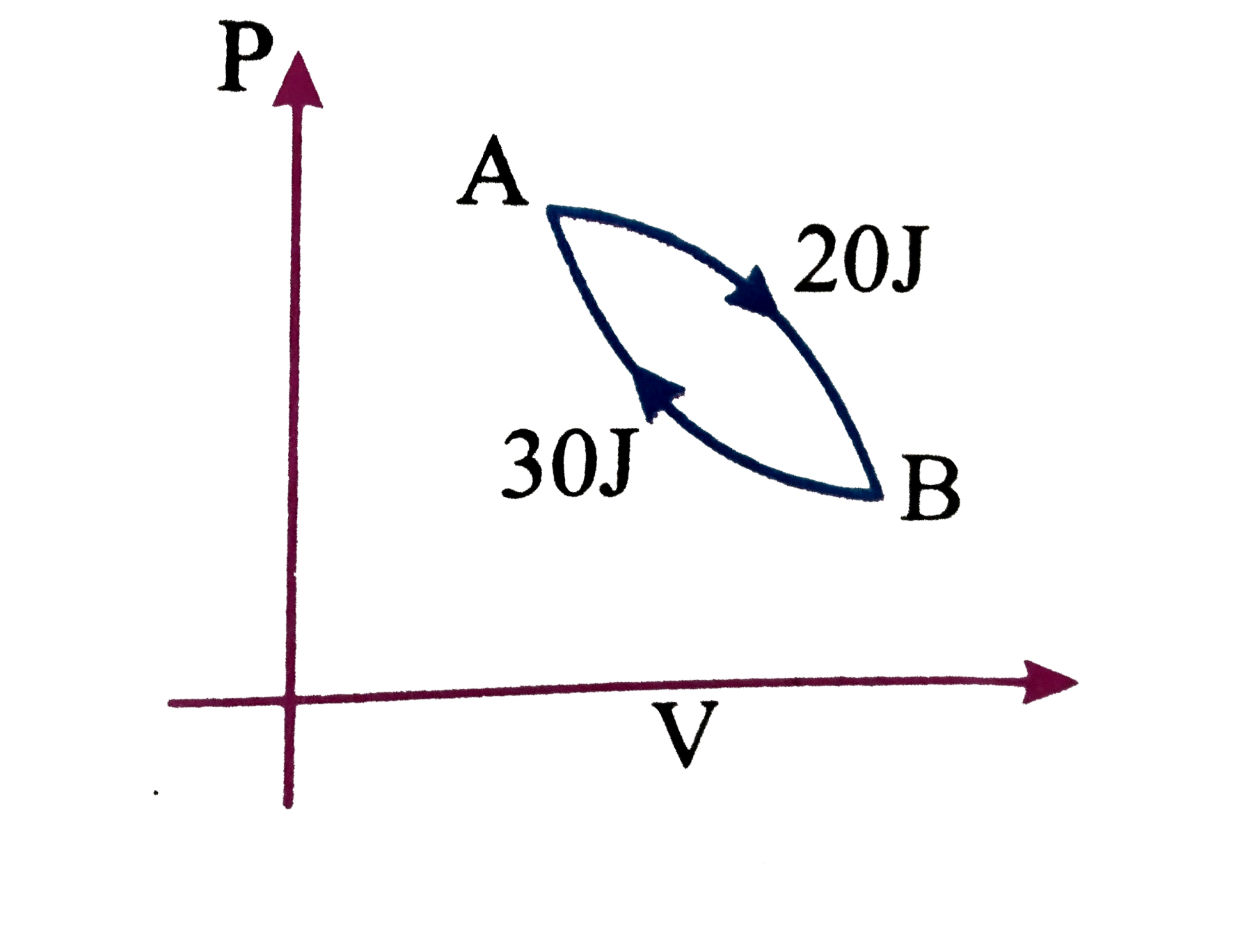
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KTG & THERMODYNAMICS
RESONANCE|Exercise SECTION (D)|2 VideosKTG & THERMODYNAMICS
RESONANCE|Exercise SECTION (I)|2 VideosKTG & THERMODYNAMICS
RESONANCE|Exercise SECTION (A)|3 VideosKINETIC THEORY OF GASES AND THERMODYNAMICS
RESONANCE|Exercise Exercise|64 VideosMAGNETIC FIELD AND FORCES
RESONANCE|Exercise Exercise|65 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-KTG & THERMODYNAMICS-SECTION
- A given quantity of a ideal gas is at pressure P and absolute tempera...
Text Solution
|
- In figure, A and B are two adiabatic curves for two different gases. T...
Text Solution
|
- In a cyclic process shown in the figure an ideal gas is adiabatically...
Text Solution
|
- In given figure, a fixed mass of an ideal gas undergoes the change rep...
Text Solution
|
- A certain mass of an ideal gas is at pressure P(1) and volume V(1). If...
Text Solution
|
- During an adiabatic process, the pressure of a gas is found to be prop...
Text Solution
|
- A gas undergoes a process in which its pressure P and volume V are rel...
Text Solution
|
- V = k((P)/(T))^(0.33) where k is constant. It is an,
Text Solution
|
- One mole of a gas is subjected to two process AB and BC, one after the...
Text Solution
|
- An ideal gas has an adiabatic exponent gamma. In some process its mola...
Text Solution
|
- One mole of an ideal gas undergoes a process in which T = T(0) + aV^(3...
Text Solution
|
- In the above question, maximum pressure attainable is
Text Solution
|
- In a certain gas, the ratio of the velocity of sound and root mean squ...
Text Solution
|
- A polytropic process for an ideal gas is represented by equation PV^(n...
Text Solution
|
- One mole of an ideal gas at temperature T expands slowly according to ...
Text Solution
|
- In a process the pressure of a gas is inversely proportional to the sq...
Text Solution
|
- The molar heat capacity for the process shown in figure is
Text Solution
|
- The coefficient of performance of a Carnot refrigerator working betwee...
Text Solution
|
- If the door of a refrigerator is kept open, then which of the followin...
Text Solution
|
- An ideal gas heat engine operates in a Carnot's cycle between 227^(@)C...
Text Solution
|