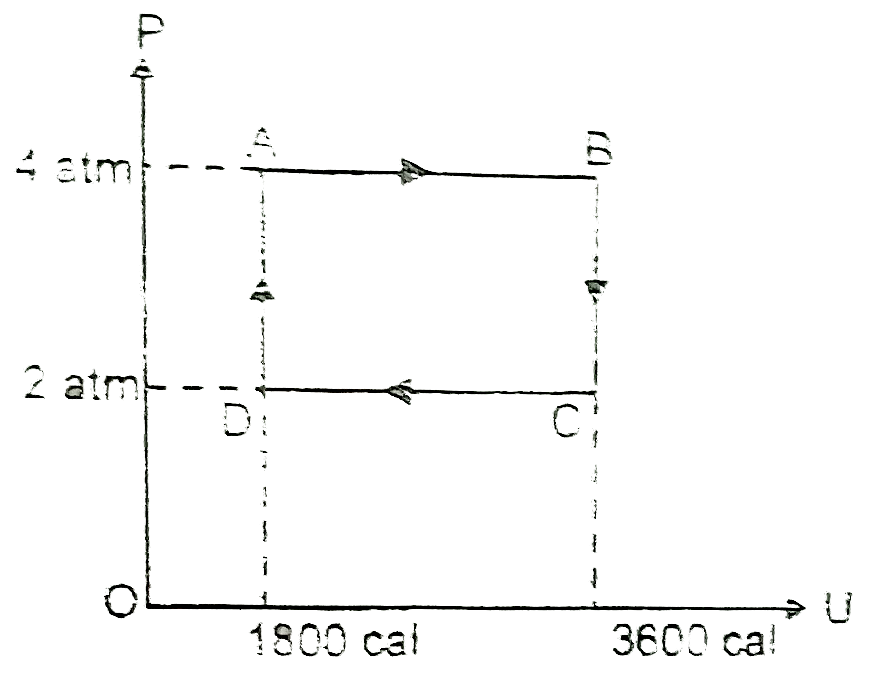
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KTG & THERMODYNAMICS
RESONANCE|Exercise Exercise-2|1 VideosKTG & THERMODYNAMICS
RESONANCE|Exercise PART -I|15 VideosKTG & THERMODYNAMICS
RESONANCE|Exercise SECTION (J)|2 VideosKINETIC THEORY OF GASES AND THERMODYNAMICS
RESONANCE|Exercise Exercise|64 VideosMAGNETIC FIELD AND FORCES
RESONANCE|Exercise Exercise|65 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-KTG & THERMODYNAMICS-PART -II
- When an ideal gas is compressed isothermally then its pressure increas...
Text Solution
|
- A vessel of volume V = 5 litre contains 1.4 g nitrogen and 0.4g of He ...
Text Solution
|
- In given figure, an ideal gas is trapped between a mercury column and ...
Text Solution
|
- Two vessels A and B, thermally insulated, contain an ideal monoatomic ...
Text Solution
|
- Consider a vertical tube open at both ends. The tube consistss of two ...
Text Solution
|
- When 2g of gas A is introduced into an evacuated flask kept at 25^(@)C...
Text Solution
|
- Two moles of an ideal monoatomic gas undergo a cyclic process which is...
Text Solution
|
- In figure, a sample of 3 moles of an ideal gas is undergoing through a...
Text Solution
|
- During the expansion process the volume of the gas changes form 4m^(3)...
Text Solution
|
- A balloon containing an ideal gas has a volume of 10 litre and tempera...
Text Solution
|
- One mole of an ideal gas is kept enclosed under a light piston (area =...
Text Solution
|
- 3 moles of an ideal mono atomic gas performs a cycle as shown in fig. ...
Text Solution
|
- An adiabatic cylindrical tube is fitted with an adiabatic separator as...
Text Solution
|
- A diatomic ideal gas undergoes a thermodynamic change according to the...
Text Solution
|
- A thermodynamic process of one mole ideal monoatomic gas is shown in f...
Text Solution
|
- P-V indicator diagram for a given sample of monoatomic idela gas is sh...
Text Solution
|
- A thermally insulated piston divides a nonconducting container in two ...
Text Solution
|