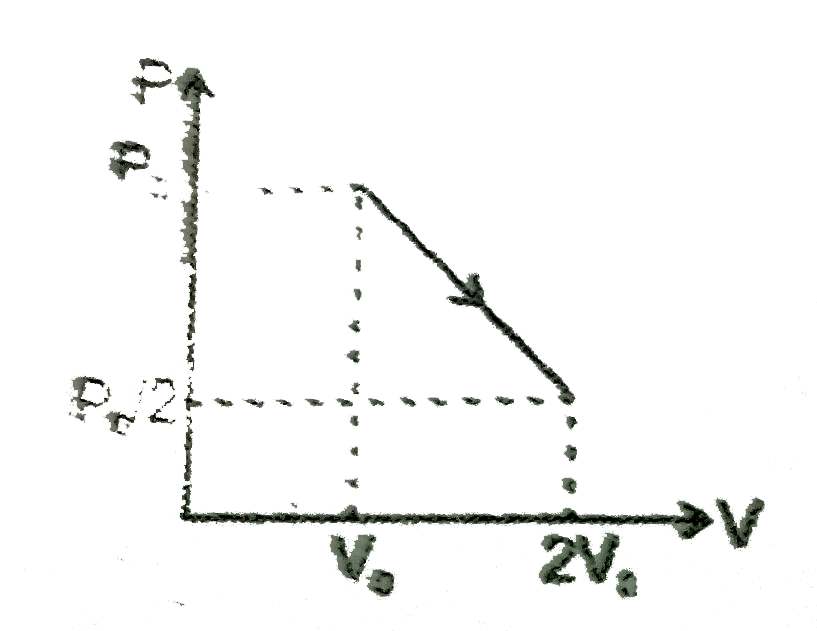
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KTG & THERMODYNAMICS
RESONANCE|Exercise PART -IV|10 VideosKTG & THERMODYNAMICS
RESONANCE|Exercise Exercise-3|1 VideosKTG & THERMODYNAMICS
RESONANCE|Exercise PART -I|15 VideosKINETIC THEORY OF GASES AND THERMODYNAMICS
RESONANCE|Exercise Exercise|64 VideosMAGNETIC FIELD AND FORCES
RESONANCE|Exercise Exercise|65 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-KTG & THERMODYNAMICS-PART -III
- Pick the correct statement (s) :
Text Solution
|
- Graph shows a hypothetical speed distribution for a sample of N gas pa...
Text Solution
|
- A system undergoes a cyclic process in which it absorbs Q(1) heat and ...
Text Solution
|
- The pressure P and volume V of an ideal gas both decreases in a proces...
Text Solution
|
- An ideal gas can be taken form initial state 1 to final state 2 by two...
Text Solution
|
- In given figure, let DeltaU(1) and DeltaU(2) be change in internal ene...
Text Solution
|
- Specific heat of a substance can be
Text Solution
|
- The following sets of values for C(v) and C(p) of an ideal gas have be...
Text Solution
|
- For an ideal gas :
Text Solution
|
- An ideal monatomic gas is at P(0), V(0). It is taken to final volume 2...
Text Solution
|
- A gaseos mixture consists of equal number of moles of two ideal gases ...
Text Solution
|
- Let n(1) and n(2) moles of two different ideal gases be mixed. If adia...
Text Solution
|
- An ideal gas can be expanded form an initial state to a certain volume...
Text Solution
|
- A cyclic process ABCD is shown is shown in the following P-V diagram. ...
Text Solution
|
- A cyclic process of an ideal monoatomic gas is shown in figure. The co...
Text Solution
|
- A gas kept in a container of finite conductivity is suddenly compresse...
Text Solution
|
- Oxygen, nitrogn and helium gas are kept in three identical adiabatic c...
Text Solution
|
- A thermally insulated chamber of volume 2V(0) is divided by a friction...
Text Solution
|
- During an experiment, an ideal gas is found to obey a condition (p^2)/...
Text Solution
|
- An ideal gas undergoes a thermodynamic cycle as shown in Fig. Which of...
Text Solution
|