Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CALORIMETRY AND THERMAL EXPANSION
RESONANCE|Exercise Exercise-2|1 VideosCALORIMETRY AND THERMAL EXPANSION
RESONANCE|Exercise Exercie-3|1 VideosCALORIMETRY AND THERMAL EXPANSION
RESONANCE|Exercise Exercise-1|1 VideosCALORIMETRY
RESONANCE|Exercise Exercise|19 VideosCAPACITOR
RESONANCE|Exercise Exercise|45 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-CALORIMETRY AND THERMAL EXPANSION-Exercise
- A piece of ice falls from a height h so that it melts completely. Only...
Text Solution
|
- A copper cube of mass 200g slides down an a rough inclined plane of i...
Text Solution
|
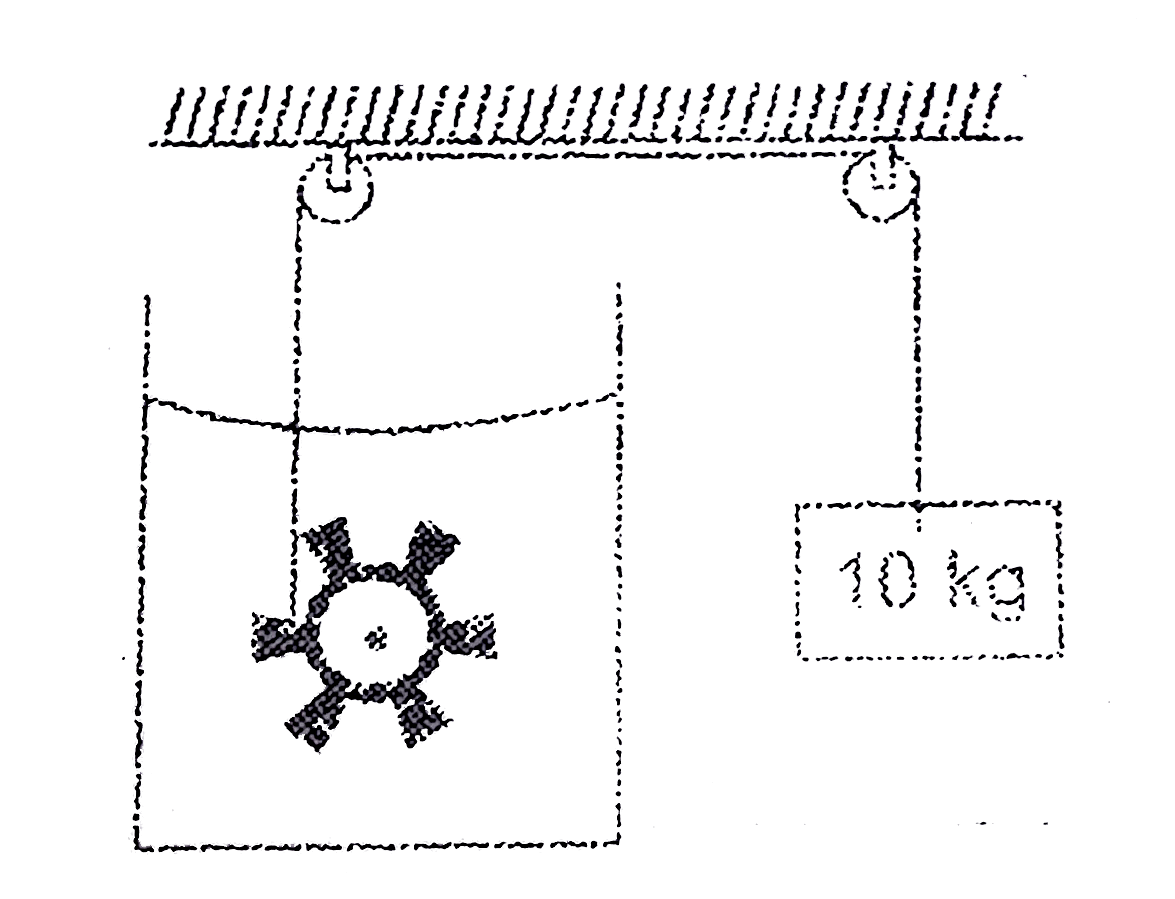
- A paddle wheel is connected with a block of mass 10kg as shown in figu...
Text Solution
|
- 300 grams of water at 25^@ C is added to 100 grams of ice at 0^@ C. Th...
Text Solution
|
- The temperature of a metal ball is raised. Arrange the percentage chan...
Text Solution
|
- A brass disc fits snugly in a hole in a steel plate. Should you heat o...
Text Solution
|
- Temperature of plate is increased by Delta theta then find new
Text Solution
|
- We have a hollow sphere and a solid sphere equal radii and of the same...
Text Solution
|
- What should be the sum of lengths of an aluminium and steel rod at 0^(...
Text Solution
|
- A steel tape is correctly calibrated at 20^(@)C and is used to measure...
Text Solution
|
- The figure shows three temperature scales with the freezing and boilin...
Text Solution
|
- At what temperature the Fahrenheit and Celsius scales of temperature g...
Text Solution
|
- A small quantity mass m, of water at a temperature theta ("in " ^(@)C)...
Text Solution
|
- A thermally isolated vessel contains 100g of water at 0^(@)C when air ...
Text Solution
|
- 20 gm ice at -10^(@)C is mixed with m gm steam at 100^(@)C. The minimu...
Text Solution
|
- 2kg ice at -20"^(@)C is mixed with 5kg water at 20"^(@)C. Then final a...
Text Solution
|
- Tow large holes are cut in a metal sheet. If this is heated, distances...
Text Solution
|
- A steel scale is to be prepared such that the millimeter intervals are...
Text Solution
|
- Expansion during heating
Text Solution
|
- If a bimetallic strip is heated it will
Text Solution
|