A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-KINETIC THEORY OF GASES AND THERMODYNAMICS-Exercise
- 12 gm He and 4 gm H(2) is filled in a container of volume 20 litre mai...
Text Solution
|
- Which of the following will have maximum total kinetic energy at tempe...
Text Solution
|
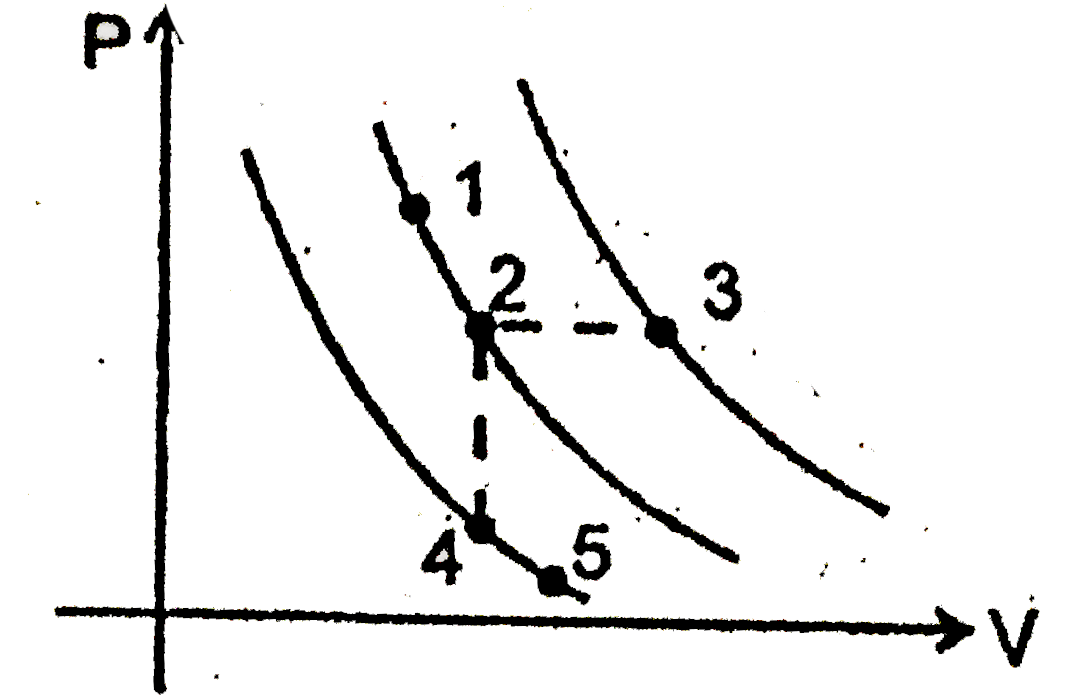
- A certain gas is taken to the five states representes by dots in the g...
Text Solution
|
- If eta=50% of oxygen gas kept in an adiabatic rigid container gets con...
Text Solution
|
- n moles of a gas are filled in a container at temperature T. If the ga...
Text Solution
|
- In a process, the pressure of an ideal gas is proportional to square o...
Text Solution
|
- A vessel contains an ideal monoatomic gas which expands at constant pr...
Text Solution
|
- In a process, the pressure of an ideal gas is proportional to square o...
Text Solution
|
- P - T diagram is shown in Fig. Choose the corresponding V - T diagram.
Text Solution
|
- A mixture of ideal gasses N(2) and He are taken in the mass ratio 14:1...
Text Solution
|
- The gas equation PV//T = constant is true for a constant mass of an id...
Text Solution
|
- DeltaU=0 in a noncylic process of an ideal gas. The process
Text Solution
|
- In an adiabatic expansion the product of pressure and volume :
Text Solution
|
- For an adiabatic process graph between PV & V for a sample of ideal ga...
Text Solution
|
- S(1): "All collisions between the molecules of the gas and walls of co...
Text Solution
|
- One mole of a mono-atomic ideal gas is mixed with one mole of a diatom...
Text Solution
|
- Hydrogen gas and oxygen gas have volume 1 cm^(3) each at N.T.P. select...
Text Solution
|
- Heat is supplied to a certain homogeneous sample of matter, at a unifo...
Text Solution
|
- An ideal gas can be expanded form an initial state to a certain volume...
Text Solution
|
- In a cyclic process, a gas is taken from state A and B via path -I as ...
Text Solution
|