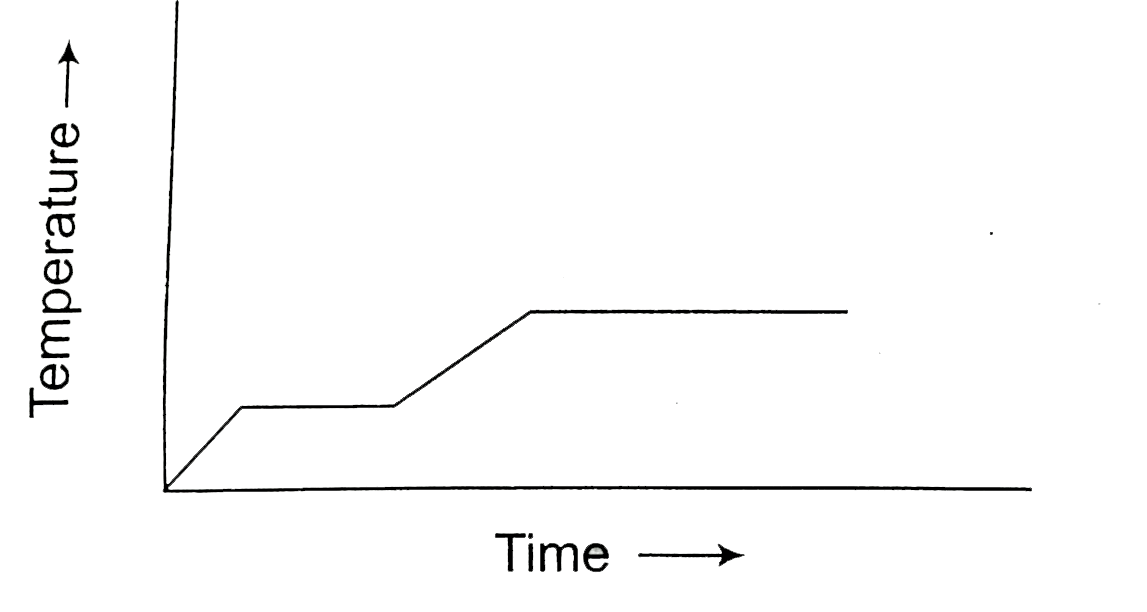
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-KINETIC THEORY OF GASES AND THERMODYNAMICS-Exercise
- One mole of a mono-atomic ideal gas is mixed with one mole of a diatom...
Text Solution
|
- Hydrogen gas and oxygen gas have volume 1 cm^(3) each at N.T.P. select...
Text Solution
|
- Heat is supplied to a certain homogeneous sample of matter, at a unifo...
Text Solution
|
- An ideal gas can be expanded form an initial state to a certain volume...
Text Solution
|
- In a cyclic process, a gas is taken from state A and B via path -I as ...
Text Solution
|
- The kinetic molecular theory of gases predicts that at a given tempera...
Text Solution
|
- On increasing the temperature, the root mean square speed of molecules...
Text Solution
|
- One of the two vessels of same capacity is filled with oxygen and oth...
Text Solution
|
- A vessel contains N molecules of a gas at temperature T. Now the numbe...
Text Solution
|
- From the molecular theory of gases, the velocity of molecules at absol...
Text Solution
|
- If the velocities of three gas molecules are sqrt(7), 4 and 5 m/s, the...
Text Solution
|
- A monoatomic gas at a temperature T has pressure P and heat energy per...
Text Solution
|
- The energy associated with each degree of freedom of a molecule
Text Solution
|
- Why does Moon have no atmosphere?
Text Solution
|
- In kinetic theory of gases, which of the following statements regardin...
Text Solution
|
- The gases carbon-monoxide (CO) and nitrogen at the same temperature ha...
Text Solution
|
- The equation of state for 5 g of oxygen at a pressure P and temperatur...
Text Solution
|
- The average kinetic energy of H(2) molecules at 300 K is E at the same...
Text Solution
|
- Which of the following statements is incorrect to assumption of kineti...
Text Solution
|
- The absolute temperature of the gas is increased 3 times. What will be...
Text Solution
|