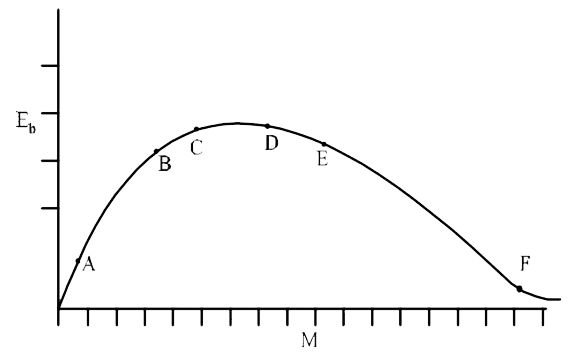
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NUCLEAR PHYSICS
RESONANCE|Exercise Exercise -3 Part-III CBSE PROBLEMS (LAST 10 YEARS)|33 VideosNUCLEAR PHYSICS
RESONANCE|Exercise Advanced level solutions|16 VideosNUCLEAR PHYSICS
RESONANCE|Exercise Exercise 3 Part -1 JEE (Advanced)|19 VideosGRAVITATION
RESONANCE|Exercise HIGH LEVEL PROBLEMS|16 VideosREVISION DPP
RESONANCE|Exercise All Questions|444 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-NUCLEAR PHYSICS-Exercise-3 Part-II JEE (MAIN)
- Starting with a sample of pure .^(66)Cu, 7//8 of it decays into Zn in ...
Text Solution
|
- If radius of the .(13)^(27)Al nucleus is estimated to be 3.6 Fermi, th...
Text Solution
|
- A nuclear transformation is denoted by X(n,alpha) rarr. (3)^(7)Li. Whi...
Text Solution
|
- The energy spectrum of beta - particle [number N€ as a function of bet...
Text Solution
|
- When (3)Li^(7) nuclei are bombarded by protons , and the resultant nuc...
Text Solution
|
- The 'rad' is the correct unit used to report the measurement of :
Text Solution
|
- If the binding energy per nucleon in .(3)^(7)Li and .(2)^(4)He nuclei ...
Text Solution
|
- If M(o) is the mass of an oxygen isotope .(8)O^(17), M(p) and M(N) are...
Text Solution
|
- In gamma ray emission from a nucleus
Text Solution
|
- The half-life period of a radio-active element X is same as the mean l...
Text Solution
|
- This question contains Statement - 1 and Statement -2 Of the four choi...
Text Solution
|
- The above is a plate of binding energy per nucleon E(0) against the nu...
Text Solution
|
- This binding energy per nucleon for the parent nucleus is E(1) and tha...
Text Solution
|
- A nuclear of mass M +deltam is at rest and decay into two daughter nu...
Text Solution
|
- A radiaoactive nucleus (initial mass number A and atomic number Z emit...
Text Solution
|
- The half life of a radioactive substance is 20 minutes . The approxima...
Text Solution
|
- Statement-1:A nucleus having energy E(1) decays by beta^(-) emission t...
Text Solution
|
- Assume that a neutron breaks into a proton and an electron . The energ...
Text Solution
|
- In a hydrogen like atom electron make transition from an energy level ...
Text Solution
|