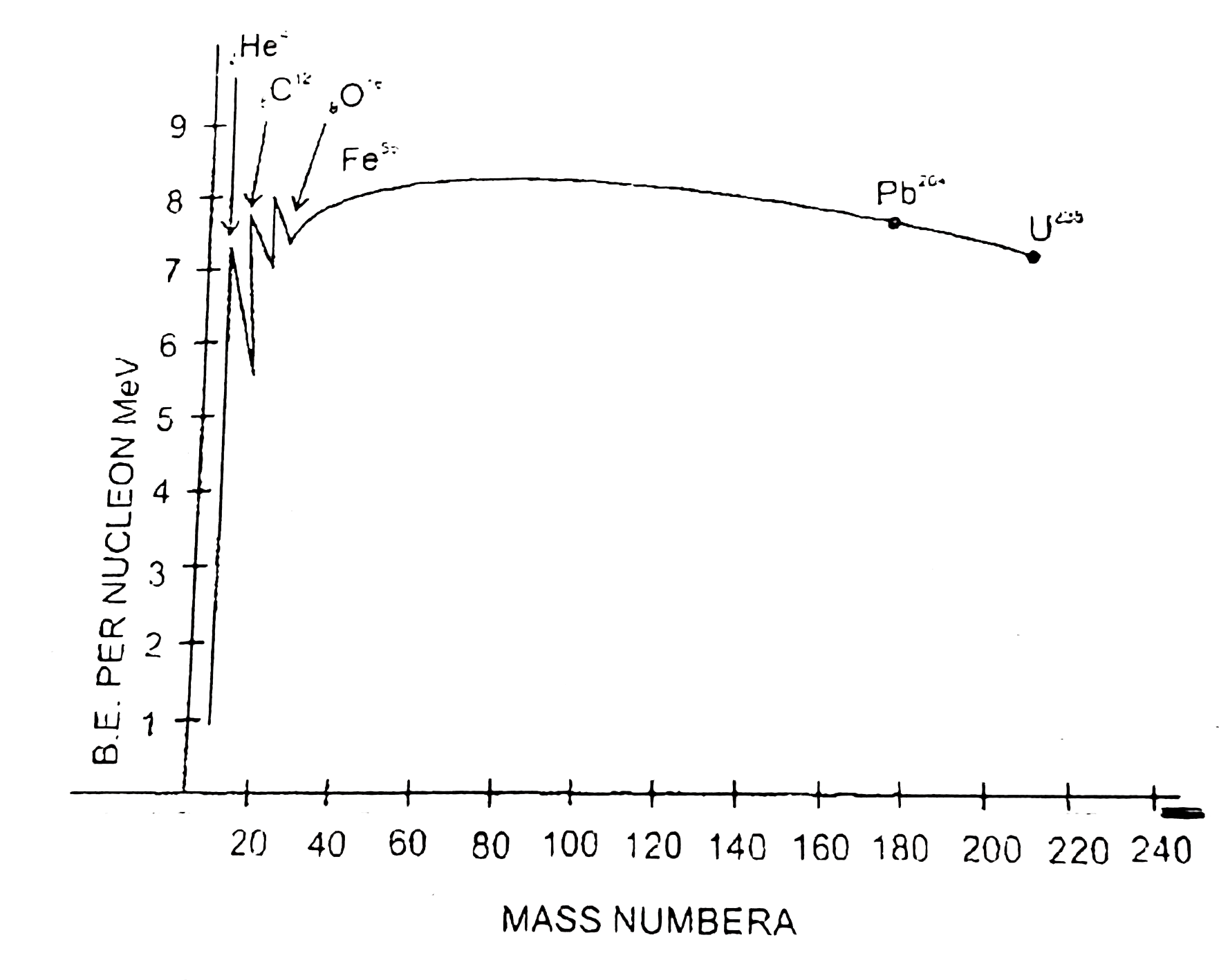
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-NUCLEAR PHYSICS-Exercise -3 Part-III CBSE PROBLEMS (LAST 10 YEARS)
- Explain the concept of nuclear forces. Discuss their characterstic pro...
Text Solution
|
- State and explain the laws of radioactive disintergration. Hence defin...
Text Solution
|
- Calculate the binding energy per nucleon of .(20)^(40)Ca. Given that ...
Text Solution
|
- Calculate the amount of energy released during the alpha-decay of .(...
Text Solution
|
- A neutron is absorbed by a .(3)^(6)Li nucleus with the subsequent emis...
Text Solution
|
- What do you understand by isotopes, isobars and isotones? Explain with...
Text Solution
|
- The nucleus .^(23)Ne deacays by beta-emission into the nucleus .^(23)...
Text Solution
|
- Two nuclie have mass numbers in the ratio 1:2. What is the ration of t...
Text Solution
|
- A radiactive nucleus 'A' undergoes a series of decays according to the...
Text Solution
|
- Define the activity of a radionuclide. Write its SI unit. Give a plot ...
Text Solution
|
- Draw a plot of potential energy of a pair of nucleons as a function of...
Text Solution
|
- (a) Write symbolically the beta-decay process of ""(15)^(32)P. (b) ...
Text Solution
|
- How is the size of nucleus experimentally determined ? Write the relat...
Text Solution
|
- State the law of redioactive decay. Plot a graph showing the number (N...
Text Solution
|
- What is the effect on neutron to proton ratio in a nucleus when (i) an...
Text Solution
|
- A nucleus undertgoes beta-decay. How does its (i) mass number (ii) at...
Text Solution
|
- How is the radius of a nucleus related to its mass number ?
Text Solution
|
- (a) Derive the law of radioavtive decay, viz. N=N(0)e^(-lambda t) ...
Text Solution
|
- (a) Why photoelectric effect cannot be explained on the basis of wave ...
Text Solution
|
- An element A decays into element C by a two-step process : A rarr B ...
Text Solution
|