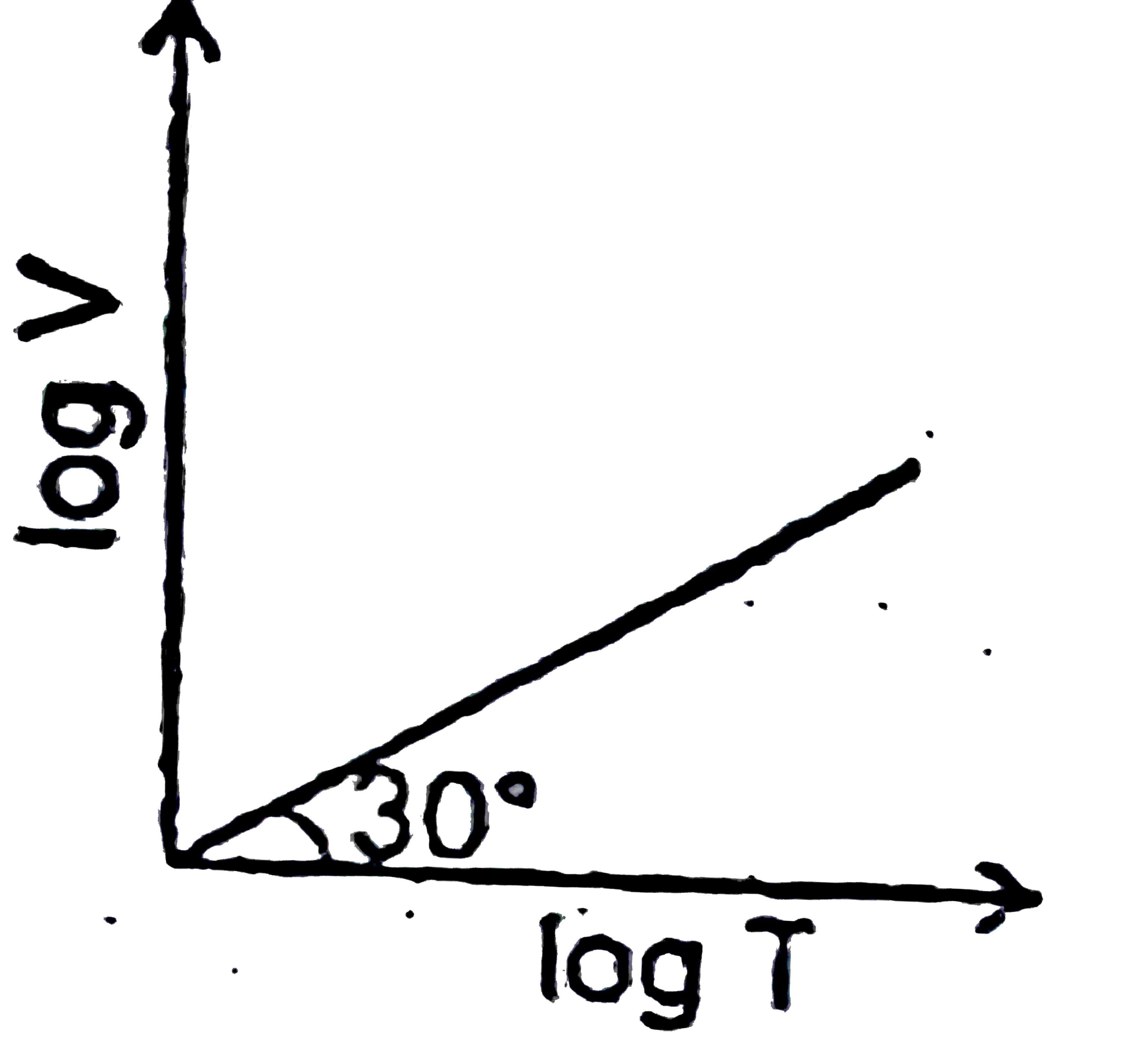
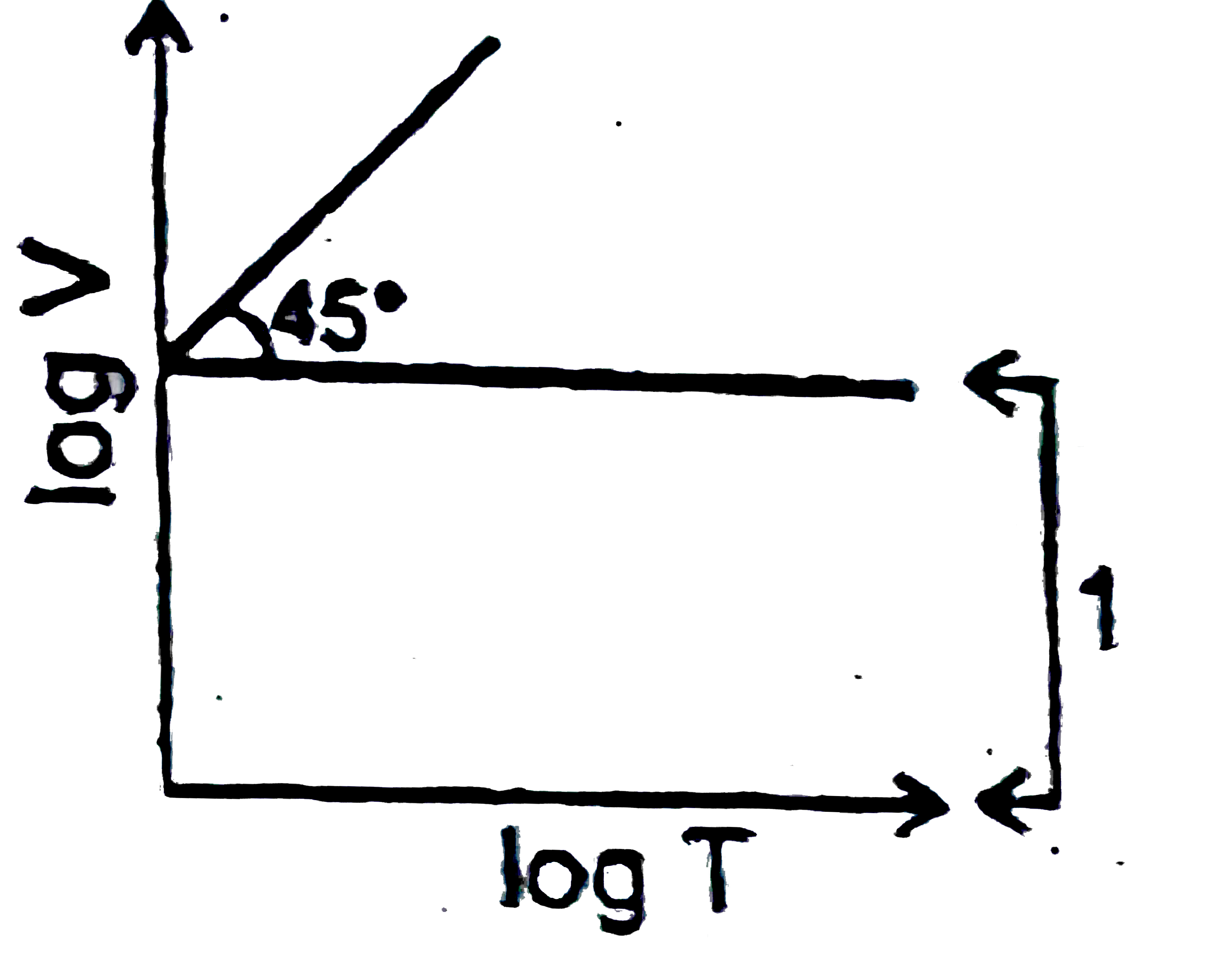
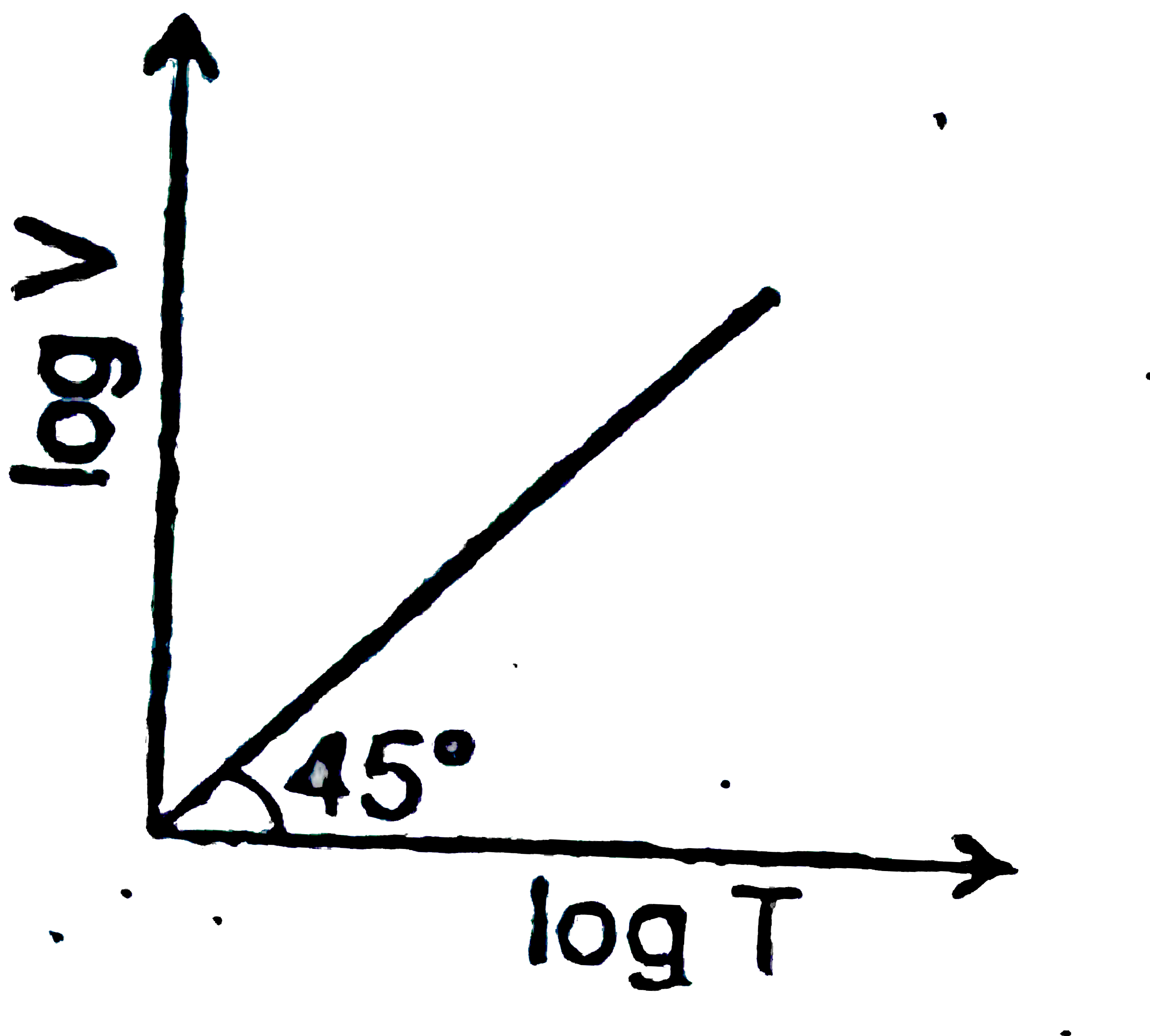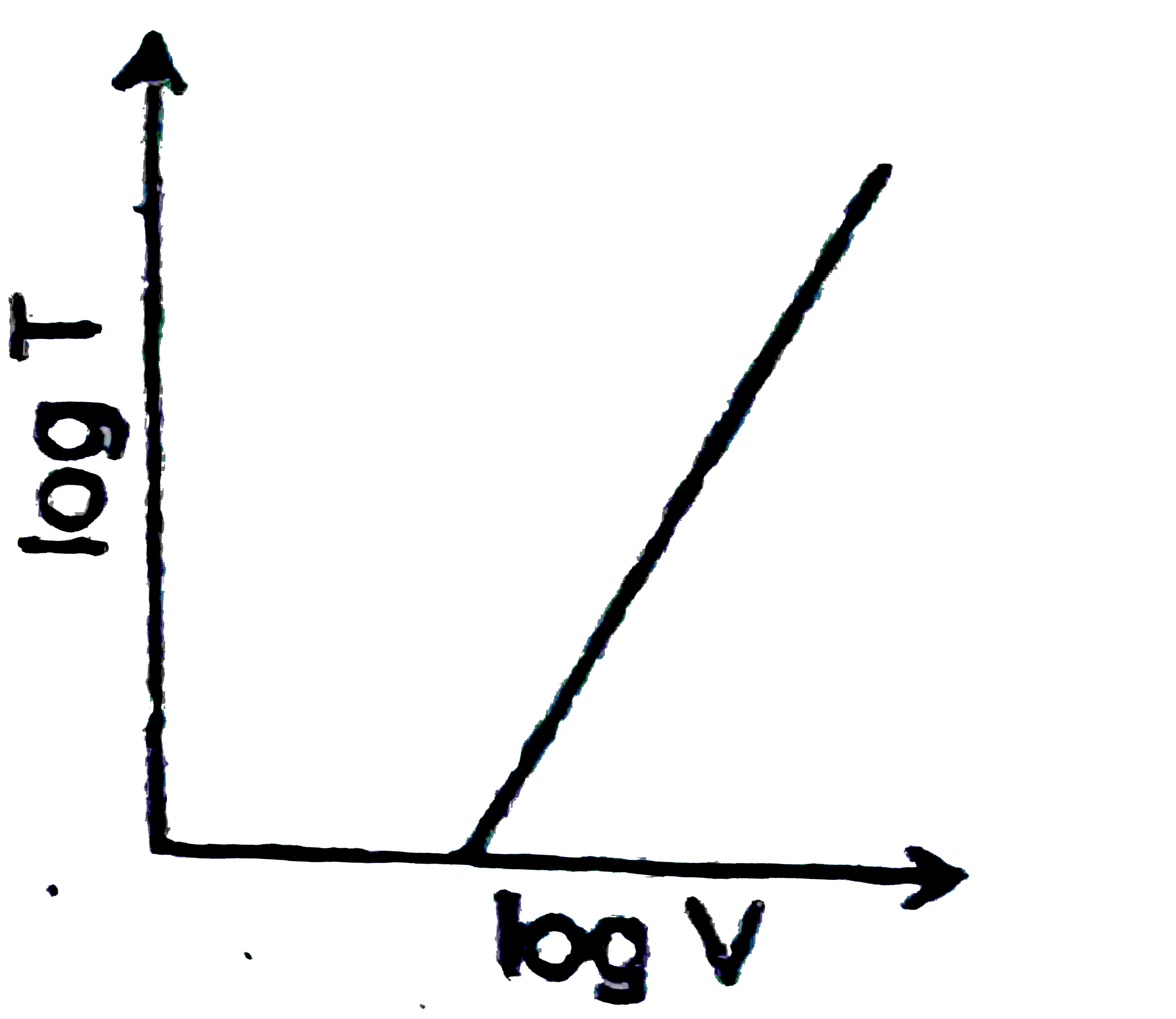A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-THERMODYNAMIC & THERMOCHEMISTRY-PHYSICAL CHEMITRY (THERMODYNAMIC & THERMOCHEMISTRY)
- A 10g piece of iron ( C =0.45 J//g^(@)C ) at 100^(@)C is dropped into ...
Text Solution
|
- An ideal gas expand against a constant external pressure at 2.0 atmosp...
Text Solution
|
- For a closed container containing 100 mol of an ideal gas fitted with ...
Text Solution
|
- 10 mole of ideal gas expand isothermally and reversibly from a pressur...
Text Solution
|
- What is the final temperature of 0.10 mole monoatomic ideal gas that p...
Text Solution
|
- During an adiabatic process, the pressure of a gas is found to be prop...
Text Solution
|
- P-V plot for two gases ( assuming ideal ) during adiabatic processes ...
Text Solution
|
- Calculate the final temperature of a monoatomic ideal gas that is comp...
Text Solution
|
- Calculate average molar that capacity at constant volume of gaseous mi...
Text Solution
|
- Consider the reaction at 300K C(6)H(6)(l)+(15)/(2)O(2)(g)rarr 6CO(2)...
Text Solution
|
- At 5xx10^(4) bar pressure density of diamond and graphite are 3 g//c c...
Text Solution
|
- Predict which of the following reaction (s) has a positive entropy cha...
Text Solution
|
- What is the change in entropy when 2.5 mole of water is heated from 27...
Text Solution
|
- Calculate standard entropy change in the reaction Fe(2)O(3)(s)+3H(2)...
Text Solution
|
- For isothermal expansion in case of an ideal gas :
Text Solution
|
- The standard enthalpy of formation of octane (C(8)H(18)) is -250kJ //m...
Text Solution
|
- Calculate P-Cl bond enthalpy Given : Delta(r)H(PCl(3),g)=306kJ //mo...
Text Solution
|
- The enthalpy of neutralization of HNO(3) by NaOH=-13680 cal//eqt. When...
Text Solution
|
- Ethyl chloride (C(2)H(5)Cl) , is prepared by reaction of ethylene with...
Text Solution
|
- Given a. NH(3)(g)+3CI(2)(g)rarr NCI(3)(g)+3HCI(g),DeltaH(1) b. N(2...
Text Solution
|