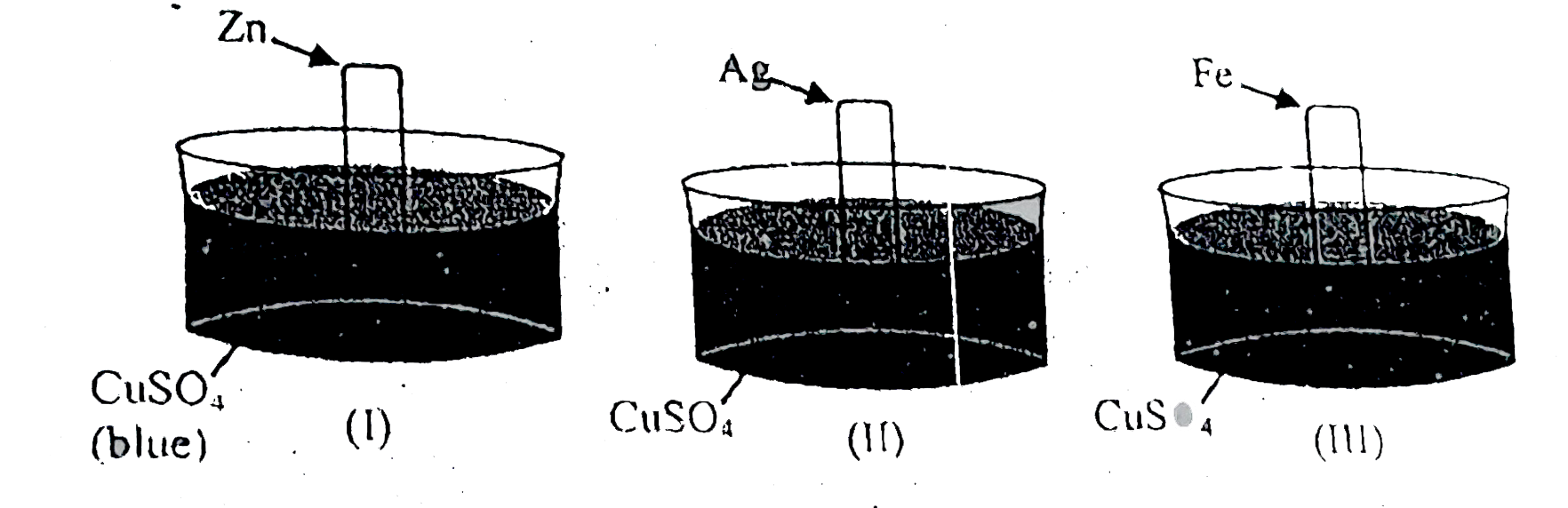
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-ELECTRO CHEMISTRY-PHYSICAL CHEMITRY (ELECTROCHEMISTRY)
- E^(@) for F(2)+2e^(-) hArr 2F^(-) is 2.8V,E^(@) for (1)/(2)F+e^(-)=F^(...
Text Solution
|
- Given that E(Fe^(2+)//Fe)^(.)=-0.44V,E(Fe^(3+)//Fe^(2+))^(@)=0.77V if...
Text Solution
|
- Consider following sets : Blue colour solution changes to colourl...
Text Solution
|
- E(Al^(3+)//Al)^(@)=-1.66 V and K(SP) of Al(OH)(3)=1.0xx10^(-33). Reduc...
Text Solution
|
- The E(Call)^(@)=1.18V for Zn(s)||Zn^(+2)(1M)||Cu^(+2)(1M)|Cu(s). The v...
Text Solution
|
- You are given the followin cell at 298 K, Zn|(Zn^(+ +) .((aq.))),(0.01...
Text Solution
|
- What must be concentration of Ag^(+) in an aqueous solution containin...
Text Solution
|
- Consider the cell potentials E(Mg^(2+)|Mg)^(0)=-2.37V and E(Fe^(3+)|Fe...
Text Solution
|
- Use the standard potentials of the couples Au^(+)//Au(+1.69V),Au^(3+)/...
Text Solution
|
- Calculate molar conductivity of HCOOH at infinite dilution, if equival...
Text Solution
|
- Salts of A (atomic weight 7), B (atomic weight 27) and C (atomic weigh...
Text Solution
|
- Given that E(Fe^(3+)|Fe)^(0) and E(Fe^(2+)|Fe)^(0) are -36V and -0.43...
Text Solution
|
- The standard oxidation potential for Mn^(3+) ion acid solution are Mn^...
Text Solution
|
- Fe is reacted with 1.0M HCI.E^(@) for Fe//Fe^(2+) = +0.34 volt. The co...
Text Solution
|
- The following facts are availabel : 2A^(c-)+B(2) rarr 2B^(-)+A(2), ...
Text Solution
|
- E(H^(+)//H(2))^(0)=0.00V, then E(D^(+)//D(2))^(0) at 25^(@)C will be
Text Solution
|
- When an electric current is passed through acidified water, 112ml of H...
Text Solution
|
- Which of the following graphs are correct for strgon electrolyte AD an...
Text Solution
|
- Number of electrons lost during electrolysis of 0.14g of N^(-3) is -(N...
Text Solution
|
- Aqueous solution of NaCl containing a small amount of MeOH ( methyl o...
Text Solution
|