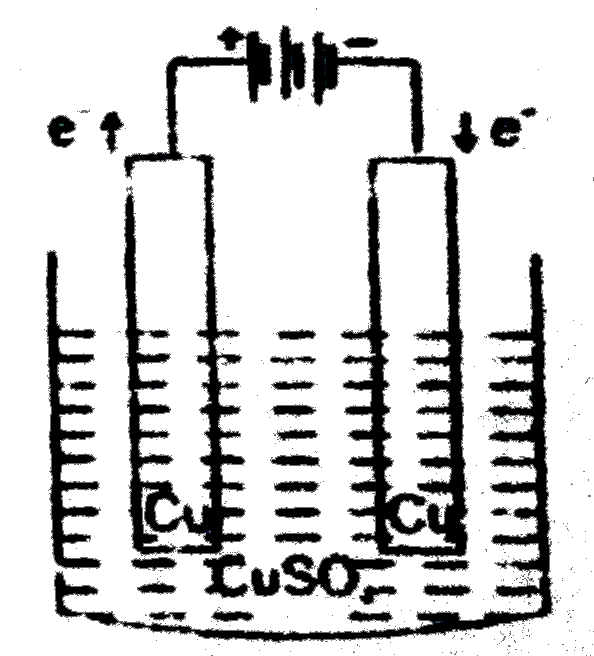A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-ELECTRO CHEMISTRY-PHYSICAL CHEMITRY (ELECTROCHEMISTRY)
- For the half cell At pH=2. Electrod potential is :
Text Solution
|
- In the electrolysis of an aqueous potassium sulphate solution, the Ph ...
Text Solution
|
- How many electrons are there in one coulomb of electricity?
Text Solution
|
- Electrolysis can be used to determine atomic masses. A current of 0.55...
Text Solution
|
- Calculate the current ( in Ma) required to deposit 0.195 g of platinum...
Text Solution
|
- An aqueous solution containing 1M each of Au^(3+),Cu^(2+),Ag^(+),Li^(...
Text Solution
|
- Based on the following information arrange four metals A,B,C and D in ...
Text Solution
|
- The standard electrode potential for the following reaction is +1.33V...
Text Solution
|
- Ag|AgCl|Cl^(-)(C(2))||Cl^(-)(C(1))||AgCl|Ag for this cell DeltaG is ne...
Text Solution
|
- Resistance of a decimolar solution between two electrodes 0.02 meter a...
Text Solution
|
- Equivalent conductivity of Fe(2)(SO(4))(3) is relative to molar condu...
Text Solution
|
- The limiting equivalent conductivity of NaCl, KCl and KBr are 126.5,1...
Text Solution
|
- The resistance of 0.1N solution of formic acid is 200 ohm and cell con...
Text Solution
|
- Given that ( ohm^(-1) cm^(2)eq^(-1)), T=298 K {:(lambda(E)^(oo) f o...
Text Solution
|
- Na- amalgam is prepared by electrolysis of NaCl solution using liquid...
Text Solution
|
- Find the thickness of the electro silver if the surface area over whic...
Text Solution
|
- In the given figure the electrolytic cell contains 1L of an aqueous 1M...
Text Solution
|
- The equilibrium Cu^(. .)(aq)+Cu(s) hArr2Cu^(.) established at 20^(@)...
Text Solution
|
- What is the cell entropy change ( in J K ^(-1)) of the following cell...
Text Solution
|
- Calculate the value of Lambda(m) ^prop for SrCl(2) in water at 25^(@)...
Text Solution
|