A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-TEST PAPERS-Physics
- On the P-T graph of an ideal gas, choose the correct option(s):
Text Solution
|
- In the figure (a) the light is incident at an angle theta (slightly gr...
Text Solution
|
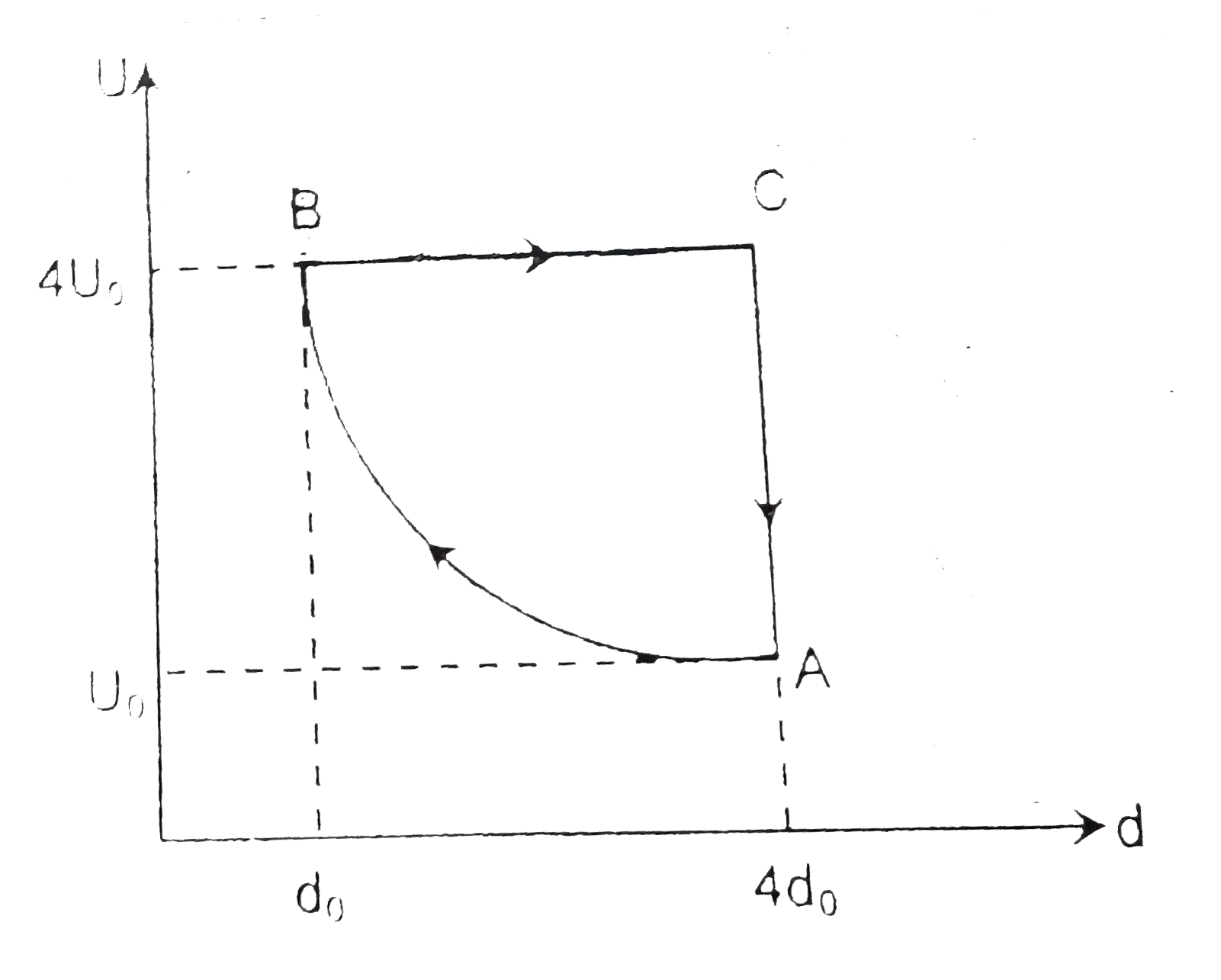
- The variation of internal energy U and density d of one mole of an ide...
Text Solution
|
- It is desired to make an achromatic combination of two lenses, (L(1)& ...
Text Solution
|
- Two identical glass slabs of same thickness are joined to form an L sh...
Text Solution
|
- An ideal monatomic gas expands in such a way that TV^((1)/(2))= consta...
Text Solution
|
- A Hydrogen gas and a helium gas each having volume 100cm^(3) at NTP ar...
Text Solution
|
- Consider one mole of an ideal gas whose volume changes with temeperatu...
Text Solution
|
- An ideal gas expands from V to 2V in 4 separate processes. Where proce...
Text Solution
|
- An object kept near a convex lens of focal length f, executes SHM betw...
Text Solution
|
- Monoatomic , diatomic and triatomic gases whose initial volume and pre...
Text Solution
|
- n moles of a monoatomic gas undergoes a cyclic process ABCDA as shown....
Text Solution
|
- Heat supplied to an ideal gas sample at constant Pressure :
Text Solution
|
- A gas is enclosed in a vessel at a constant temperature at a pressure ...
Text Solution
|
- A converging mirror forms real image of object AB on screen. Now a hol...
Text Solution
|
- When object O moves towards a fixed lens mirror combination, select co...
Text Solution
|
- Pressure versus temperature graph of an ideal gas of equal number of m...
Text Solution
|
- A ray of light is incident normally on one face of a prism as shown in...
Text Solution
|
- There are three optical media 1,2 and 3 with their refractive index mu...
Text Solution
|
- The concave and convex surface of a thin concavo-convex lens of refrac...
Text Solution
|