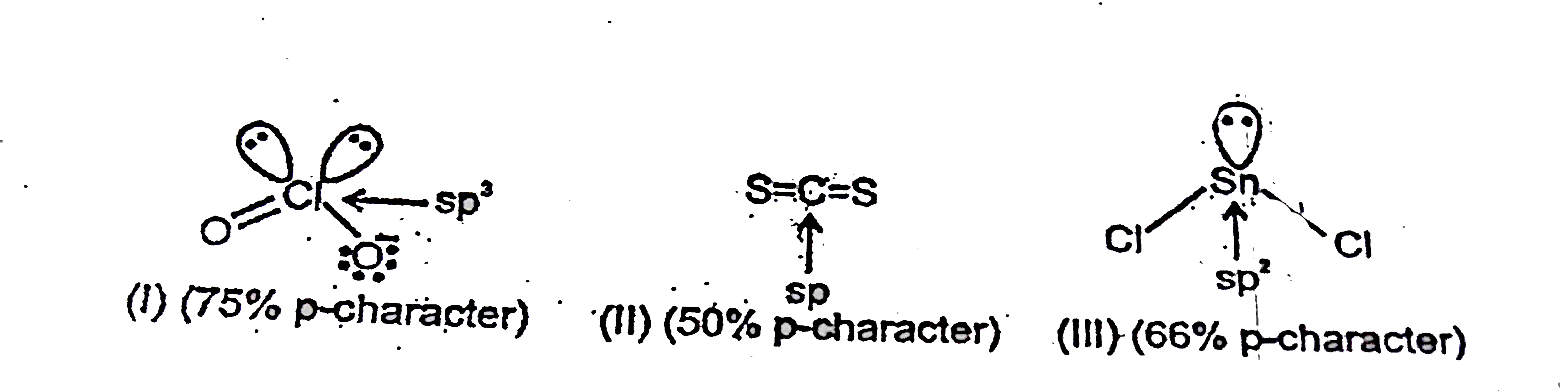A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-CHEMICAL BONDING-Inorganic chemistry (Chemistry Bonding)
- The correct order of stability to form ionic compounds among Si^(4+)...
Text Solution
|
- The correct order of hybridisation of the central atom in the followin...
Text Solution
|
- Which of the following arrangements is correct on the basis of the inc...
Text Solution
|
- Which of he following are isostructural ? {:(NO(2)^(-)",",,CO(3)^(2-...
Text Solution
|
- Which of the following is a planar molecule ?
Text Solution
|
- Which one of the following represents the INCORRECT decreasing order o...
Text Solution
|
- The number of P-O-P bonds in the tricyclic metaphophoric acid is :
Text Solution
|
- In which of the following molecule// ion all the bonds are equal?
Text Solution
|
- Which of the following options with respect to increasing bond order i...
Text Solution
|
- The boiling point of C Cl(4) higher than that of CHCl(3) because :
Text Solution
|
- Which of the following statements are correct ? (I) In ICl(2)-CIF(3)...
Text Solution
|
- Which of the following species shows intramolecular hydrogen bonding ?
Text Solution
|
- Anhydroug A Cl(3) is covalent . From the data given below Lattice En...
Text Solution
|
- In terms of the molecular orbital theory , which of the following spec...
Text Solution
|
- Which of the following statements are correct? (I) N(2)H(4) is pyra...
Text Solution
|
- Which of the following statement is // are true ?
Text Solution
|
- The maximum number of atoms which lie in the same plane in B(2)H(6) mo...
Text Solution
|
- Arrange the following compounds in increasing order of their ionic cha...
Text Solution
|
- The shape of SF(5) can be :
Text Solution
|
- In terms of polar character, which of the following order is correct ?
Text Solution
|