A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-CHEMICAL BONDING-Inorganic chemistry (Chemistry Bonding)
- In terms of the molecular orbital theory , which of the following spec...
Text Solution
|
- Which of the following statements are correct? (I) N(2)H(4) is pyra...
Text Solution
|
- Which of the following statement is // are true ?
Text Solution
|
- The maximum number of atoms which lie in the same plane in B(2)H(6) mo...
Text Solution
|
- Arrange the following compounds in increasing order of their ionic cha...
Text Solution
|
- The shape of SF(5) can be :
Text Solution
|
- In terms of polar character, which of the following order is correct ?
Text Solution
|
- O(2)F(2) is an unstable yellow change solid and H(2)O(2) is a colourle...
Text Solution
|
- The state of hybridisation of central atom in dimer of BH(3) and BeH(2...
Text Solution
|
- "Solubility of alkali metal fluorices increases down the group " Selec...
Text Solution
|
- The ratio of sigma- bond and pi- bond in tetracryano ethylene is :
Text Solution
|
- The correct order of strength of H- bond in the following compound :
Text Solution
|
- In which of the following metal to metal bond is present ?
Text Solution
|
- What is not true about ice ?
Text Solution
|
- Give the correct order of initials T or F for following statements. Us...
Text Solution
|
- Consider the following statements: I. A sigma(sigma) bond is formed ...
Text Solution
|
- Match list I with List II and select the correct answer using the code...
Text Solution
|
- Match List-I( Hybridisation) with List -II( shapes ) and select the co...
Text Solution
|
- Identify the correct sequence of increasing of pi- bonds in the struct...
Text Solution
|
- The hybridization of the central atom will change when :
Text Solution
|
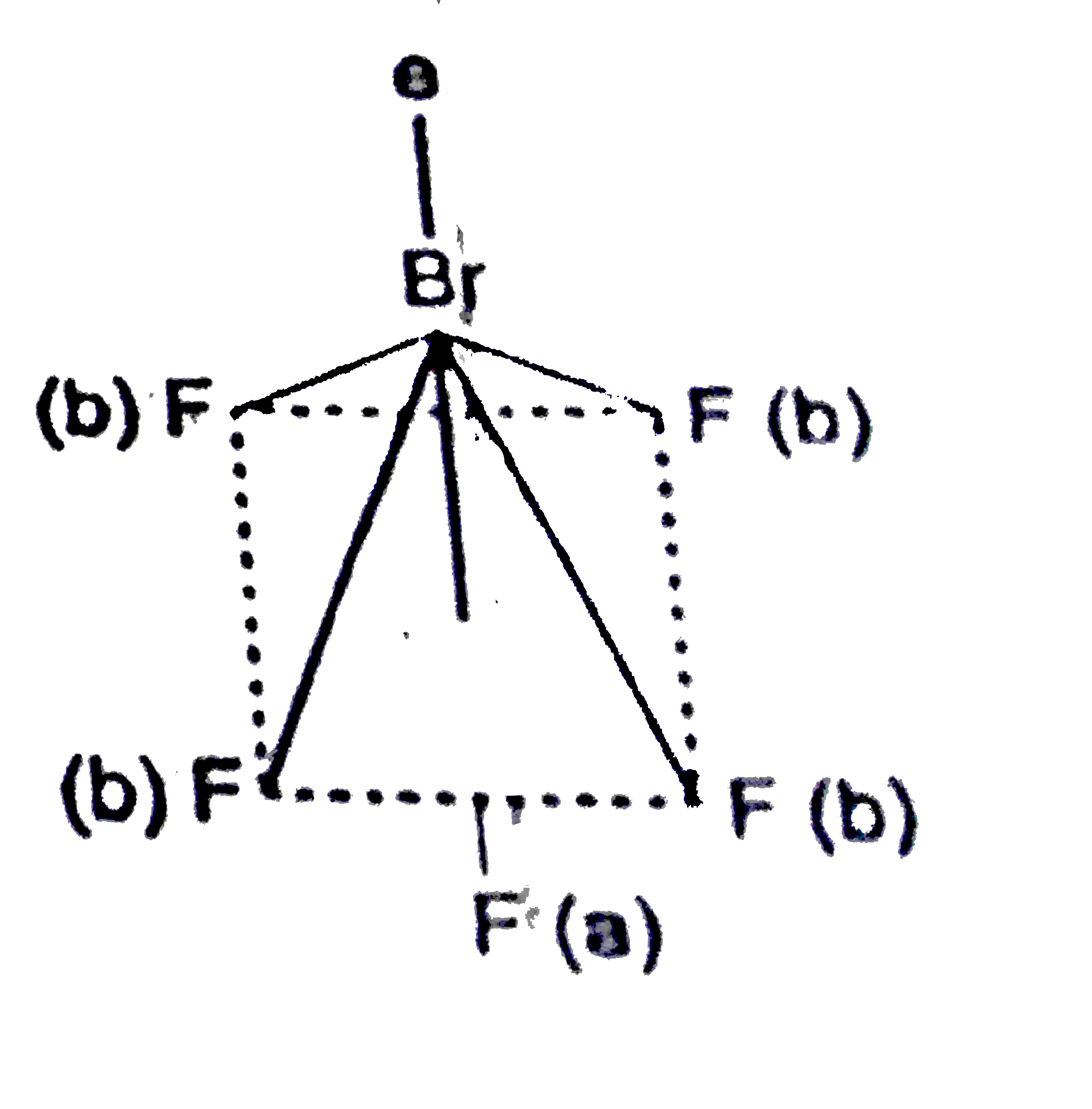
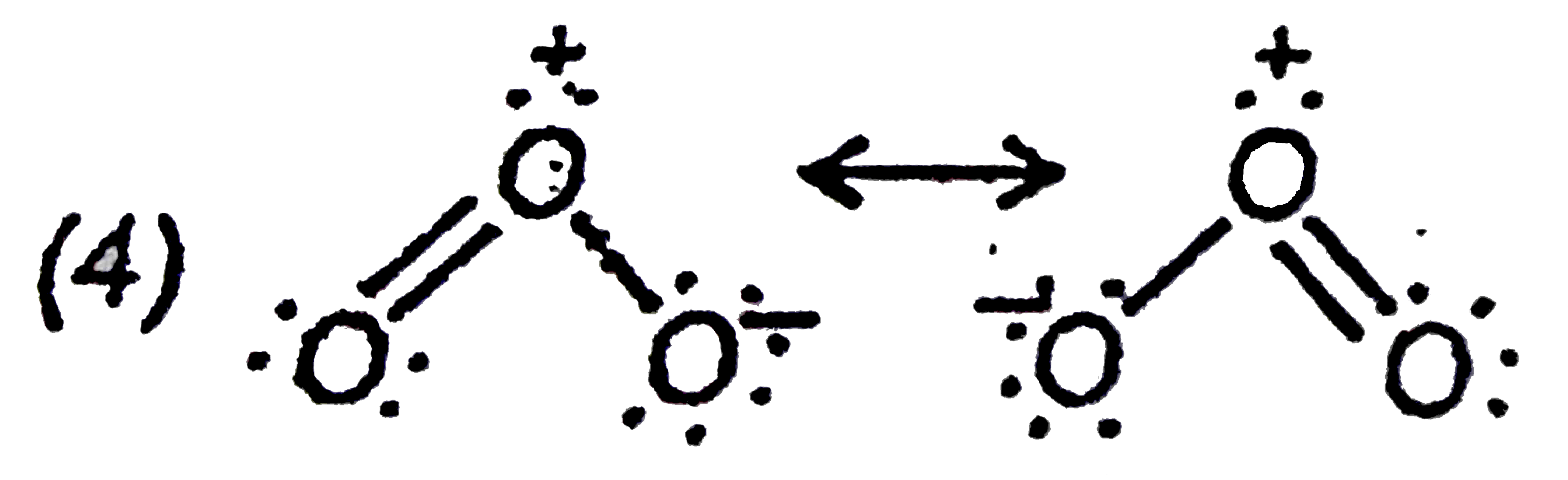 both bonds are equally stronger due to resonance.
both bonds are equally stronger due to resonance.