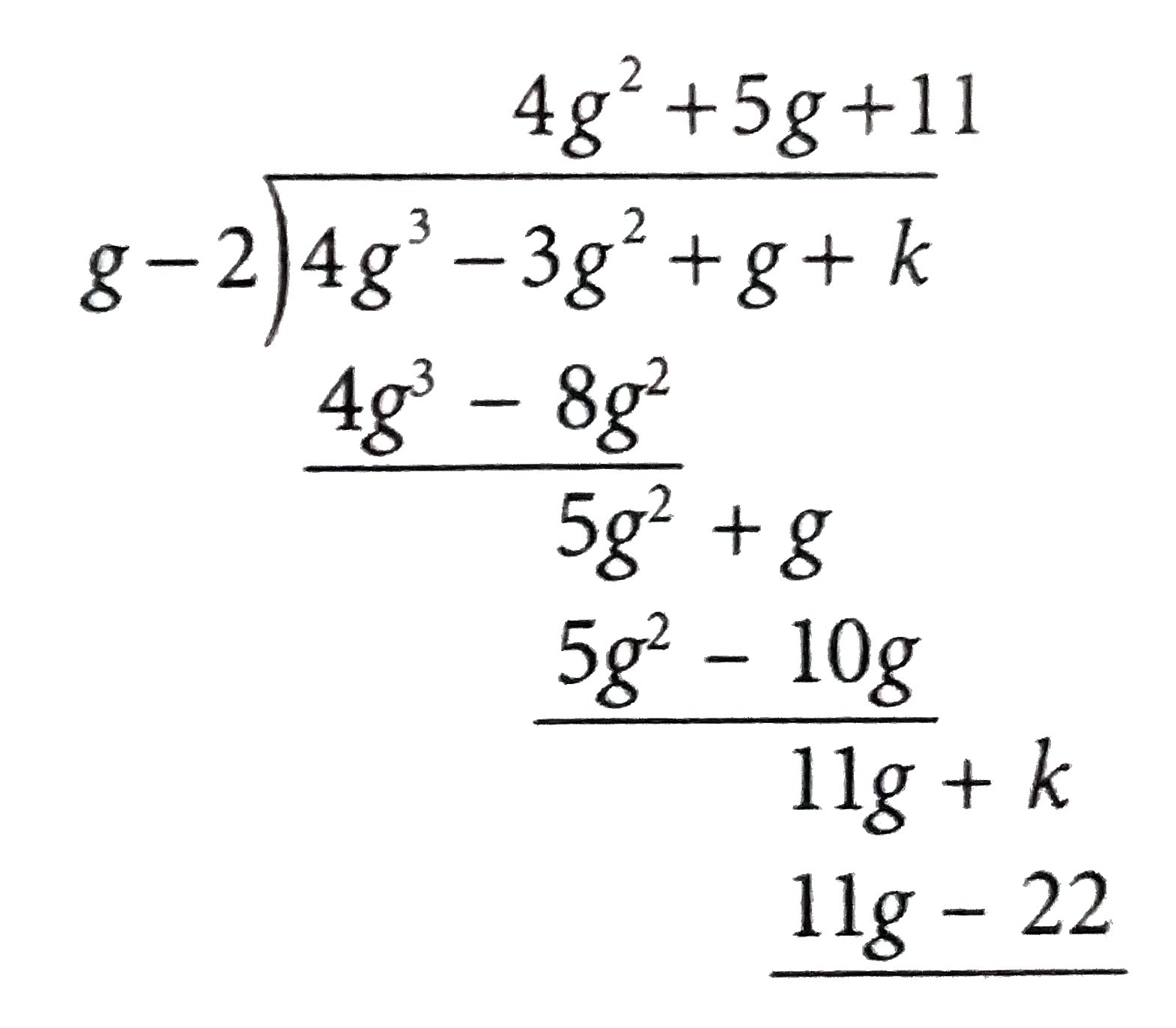A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KAPLAN-ALGEBRA -ALGEBRA FOLLOW - UP TEST
- When 4g^(3)-3g^(2)+g+k is divided by g-2, the remainder is 27. What is...
Text Solution
|
- If a = 6 - b^(3) and b = - 2, what is the value of a ?
Text Solution
|
- For all z, 3^(z)+3^(z)+3^(z) =
Text Solution
|
- For all c ne pm (1)/(5), (5c^(2)-24c-5)/(1-25c^(2))=
Text Solution
|
- When 2f^(3)+3f^(2)-1 is divided by f+2, the remainder is
Text Solution
|
- If root(5)((g-1)/(4))=(1)/(3), then g =
Text Solution
|
- If (37)/(4 sqrt(j)-19)=(37)/(17), then j =
Text Solution
|
- If 5^(k^(2))(25^(2k))(625)=25 sqrt(5) and k lt - 1, what is the value ...
Text Solution
|
- If n ne 3p, and s = (n^(2)+p)/(n-3p), what is the value of p in terms ...
Text Solution
|
- Which of the following can be a solution to the pair of equations 2q-r...
Text Solution
|
- How many integers are in the solution set |2x+6|lt (19)/(2) ?
Text Solution
|