A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-TEST PAPERS-Physics
- A uniform conducting ring of mass pi kg and radius 1 m is kept on smoo...
Text Solution
|
- A uniform conducting ring of mass pi kg and radius 1 m is kept on smoo...
Text Solution
|
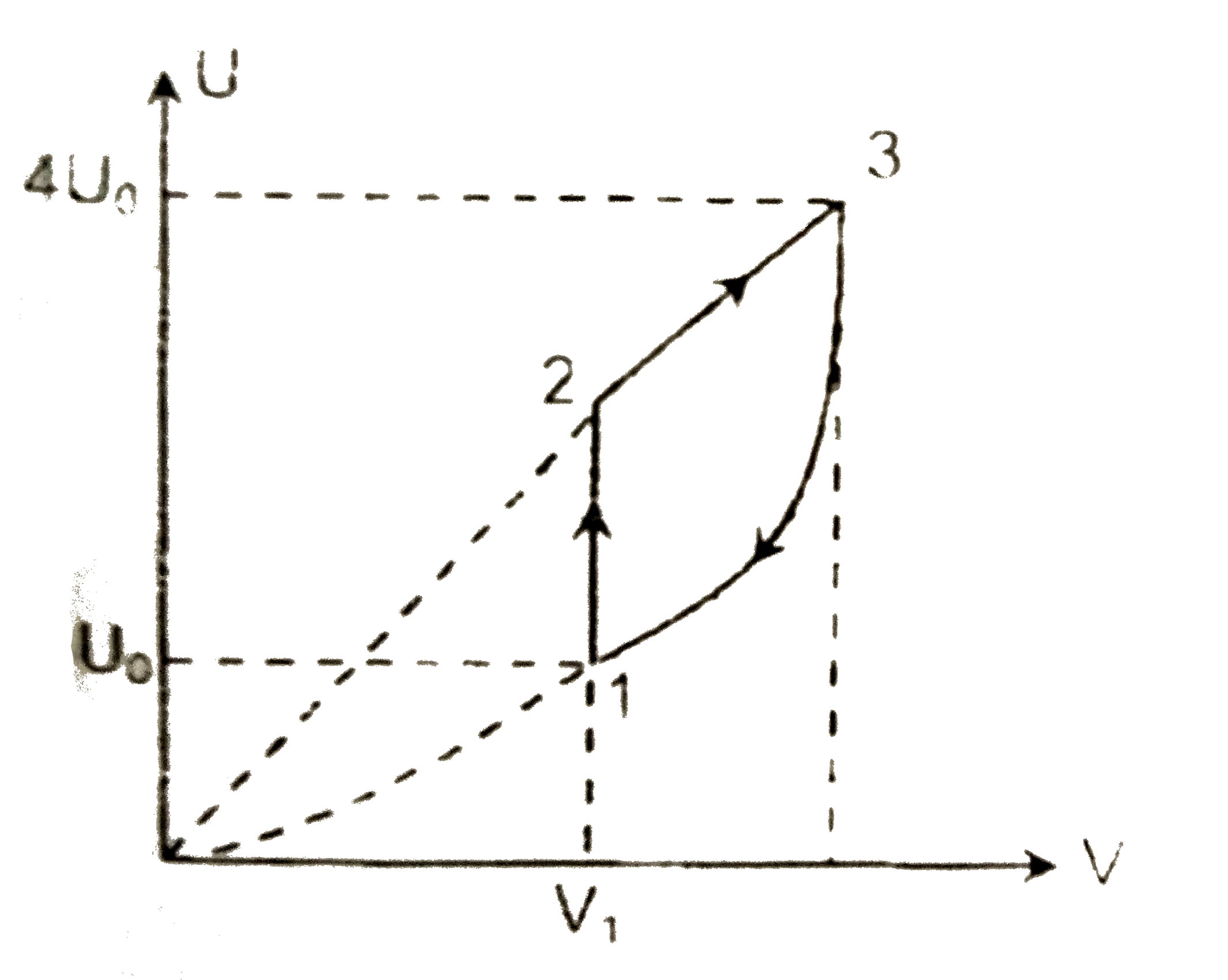
- One mole of helium gas follow cycle 1-2-3-1 shown in the diagram. Duri...
Text Solution
|
- One mole of helium gas follows the cycle 1-2-3-1 shown in the diagram....
Text Solution
|
- Bottom of a cylindrical container is made of a thin equiconvex lens ha...
Text Solution
|
- Assume that a frictionless tunnel is made in the earth along its diame...
Text Solution
|
- Consider a long cylindrical wire carrying current along the axis of wi...
Text Solution
|
- An open organ pipe in vibrating in its fifth overtone. The distance be...
Text Solution
|
- A uniform rod of mass 3 m and length l is lying on a smooth horizontal...
Text Solution
|
- A ring of mass m and radius r rolls without slipping on a fixed hemisp...
Text Solution
|
- When the voltage applied to an X-ray tube increased from V(1)=15.5kV t...
Text Solution
|
- Eight identical resistance each 15Omega are connected along the edge o...
Text Solution
|
- Two strings A and B are connected together end to end as shown in the ...
Text Solution
|
- A spherical tank of radius 0.35m is half filled with oil of relative d...
Text Solution
|
- Observer A is at rest. Source S(1) is moving towards observer A and so...
Text Solution
|
- A block of mass 1 kg is given horizontal velocity v(0)=10sqrt(3)(m)/(s...
Text Solution
|
- There is an infinite line of uniform linear density of charge +lamda. ...
Text Solution
|
- In the adjacent figure, a uniform cube of mass m and edge l=2m is lyin...
Text Solution
|
- A time varying voltage is applied across A & B such that voltage acros...
Text Solution
|
- In a binary star system one of the stars has mass equal to mass of sun...
Text Solution
|