A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS
RESONANCE|Exercise ALP PART 1 OBJECTIVE Assertion/ Reasonig|6 VideosP BLOCK ELEMENTS
RESONANCE|Exercise ALP PART 1 Comprehension # 1 OBJECTIVE|8 VideosP BLOCK ELEMENTS
RESONANCE|Exercise ALP PART CBSC- III SUBJECTIVE|1 VideosNUCLEAR CHEMISTRY
RESONANCE|Exercise STAGE-II|1 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3|8 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-P BLOCK ELEMENTS-ALP PART 1 OBJECTIVE
- Amongest H(2)O,H(2)S,H(2)Se and H(2) Te the one with highest boiling ...
Text Solution
|
- The number of S-S bonds, in sulpher trioxide trimer (S(3)O(9)) is :
Text Solution
|
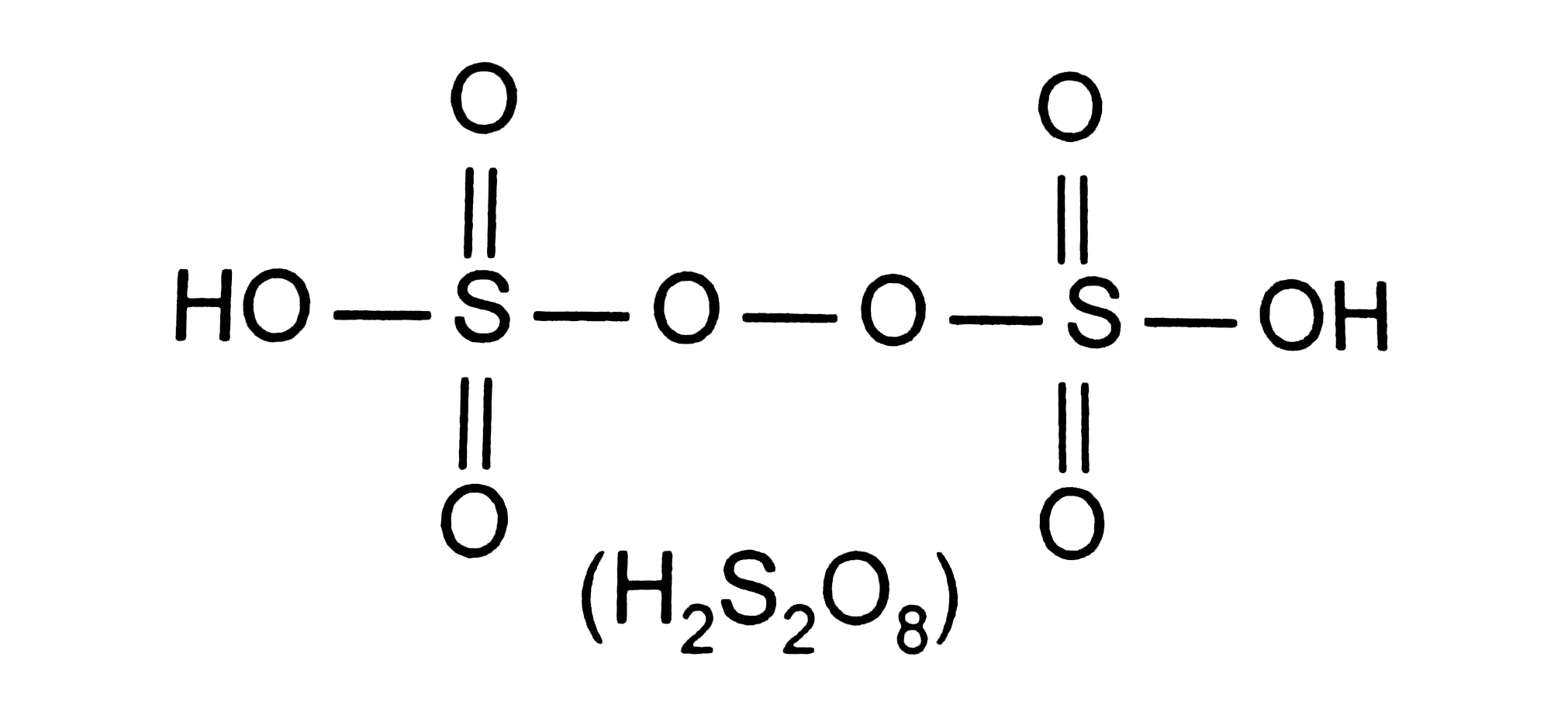
- Which of the following oxoacids of sulpher has -O-O- linkage ?
Text Solution
|
- Which of the following will not be oxidised by O(3)?
Text Solution
|
- Which gas is evolved when PbO(2) is treated with conc HNO(3) ?
Text Solution
|
- Aqueous solution of Na(2)S(2)O(3) on reaction with CI(2), gives
Text Solution
|
- Assign a reason for each of the following : (I) SCl(6) is not known ...
Text Solution
|
- Bismuth is a strong oxidizing agent in the pentavalent state. Or penta...
Text Solution
|
- H(3)PO(3) is diprotic (or dibasic). Why ?
Text Solution
|
- How will you account for the following ? Bi(2)O(3) is not acidic in ...
Text Solution
|
- Give reasons for the following observations : Why SF(4) undergoes hy...
Text Solution
|
- Why do nitro compounds have high boiling points in comparison with oth...
Text Solution
|
- Write the structure of the following species :
Text Solution
|
- Assign a reason for each of the following statements : (i) Ammonia i...
Text Solution
|
- The electron gain enthalpy with negative sign for oxygen (-141 Kj mol^...
Text Solution
|
- Bi(V) and Sb(V) which may be a stronger oxidizing agent and why ?
Text Solution
|
- Complete the following chemical reaction equations : (i)P (4(s)) +Na...
Text Solution
|
- Account for the following : (i) NH(3) is a stronger base than PH(3) ...
Text Solution
|
- Though nitrogen exhibits +5oxidation state,it does not form pentahalid...
Text Solution
|
- (a) Why does NO2 dimerise ? (b) In what way can it be proved that PH...
Text Solution
|