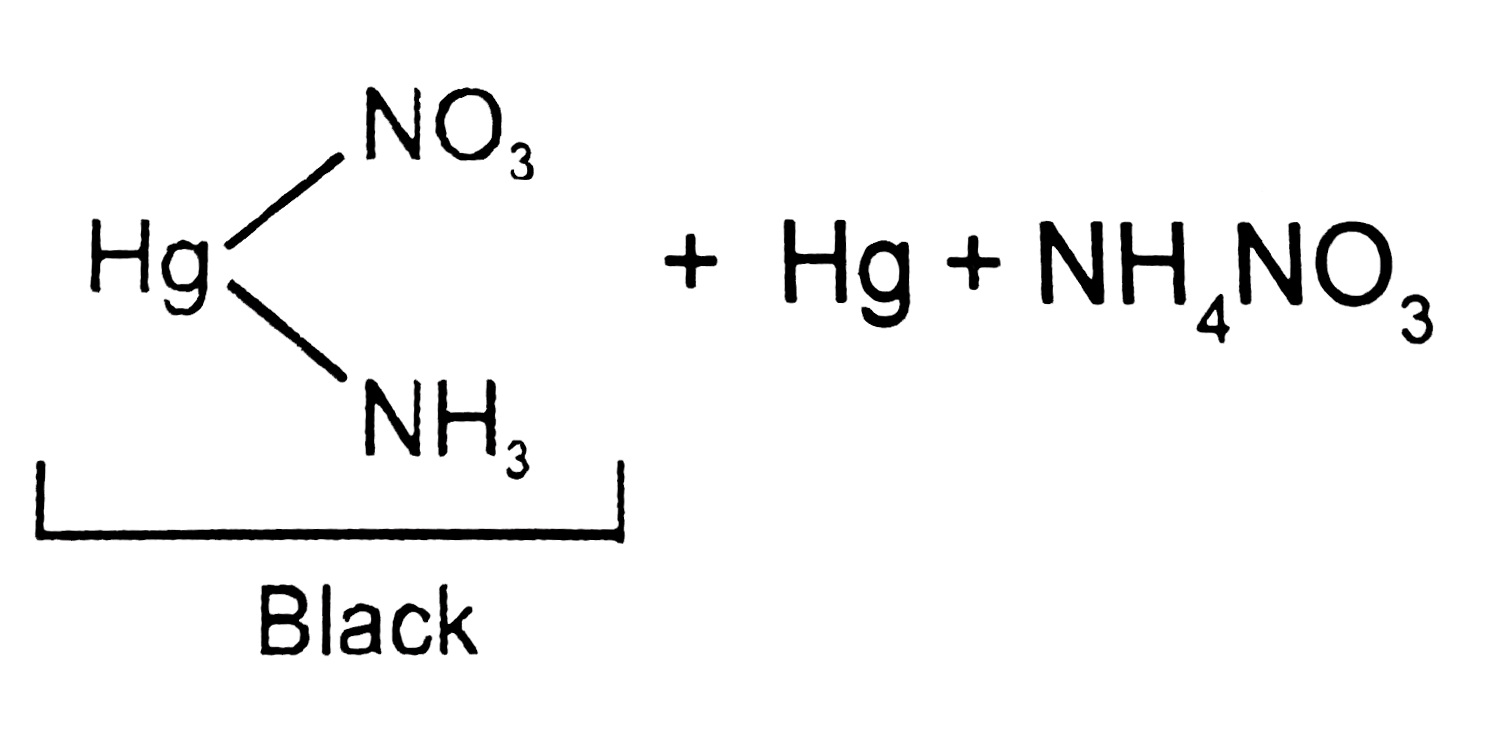A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS
RESONANCE|Exercise ALP PART 1 Comprehension # 2 OBJECTIVE|1 VideosP BLOCK ELEMENTS
RESONANCE|Exercise ALP PART 1 Comprehension # 3 OBJECTIVE|1 VideosP BLOCK ELEMENTS
RESONANCE|Exercise ALP PART 1 OBJECTIVE Assertion/ Reasonig|6 VideosNUCLEAR CHEMISTRY
RESONANCE|Exercise STAGE-II|1 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3|8 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-P BLOCK ELEMENTS-ALP PART 1 Comprehension # 1 OBJECTIVE
- Fifth group elements form hydrides to type AH(3). The hydrides have a ...
Text Solution
|
- Fifth group elements form hydrides to type AH(3). The hydrides have a ...
Text Solution
|
- Fifth group elements form hydrides to type AH(3). The hydrides have a ...
Text Solution
|
- An orange solid (A) on heating gives a green residue (B), a colourless...
Text Solution
|
- An orange solid (A) on heating gives a green residue (B), a colourless...
Text Solution
|
- Ozone is an unstable, dark blue diamagnetic gas. It absorbs strongly t...
Text Solution
|
- Ozone is an unstable, dark blue diamagnetic gas. It absorbs strongly t...
Text Solution
|
- Na(2)S(3)+2Ooverset(Delta)to Na(2)SO(4)+2S.
Text Solution
|