Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS
RESONANCE|Exercise ALP PART OBJECTIVE|12 VideosP BLOCK ELEMENTS
RESONANCE|Exercise B.L.E|49 VideosP BLOCK ELEMENTS
RESONANCE|Exercise ALP PART II SUBJECTIVE|1 VideosNUCLEAR CHEMISTRY
RESONANCE|Exercise STAGE-II|1 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3|8 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-P BLOCK ELEMENTS-ALP PART 1 Comprehension # 1 SUBJECTIVE
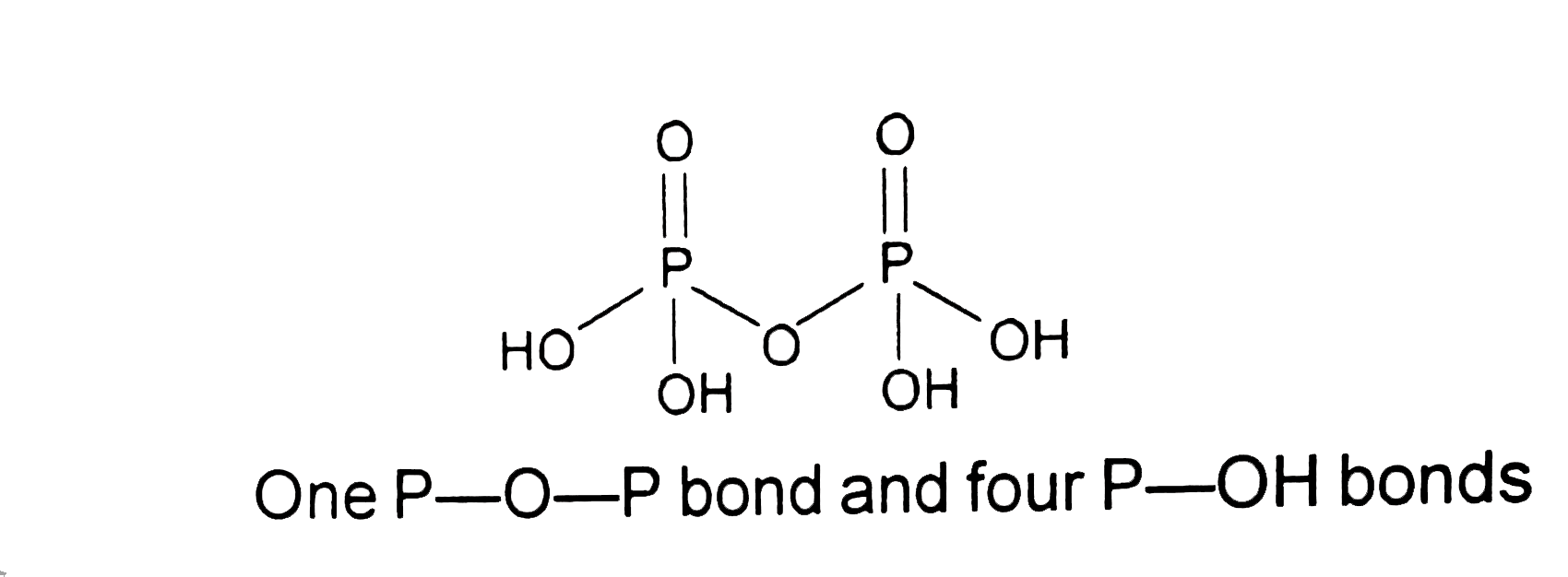
- In P(4)O(10) the number of oxygen atoms bonded to each phosphorus atom...
Text Solution
|
- Write the names of substances which have higher oxidation potential th...
Text Solution
|
- Why sulphur is able to show oxidation state of +4 and +6 with fluroine...
Text Solution
|
- Oxygen exists as a gas, while sulphur exists as a solid Why ?
Text Solution
|
- How is the presence of SO2 detected?
Text Solution
|
- Which aeroslos deplete ozone ?
Text Solution
|
- Oxygen almost variably and oxidation state of -2 but the other members...
Text Solution
|
- An aqueous solution of a gas (X) gives the following reactions : (i)...
Text Solution
|
- On heating rhombic sulphur it melts but viscosity of liquid increases ...
Text Solution
|
- {:(,underset(("Oxy acids of phosphoros"))("Column I"),,underset(("Char...
Text Solution
|
- {:("Column I","Column II"),((A)PbO(2)+HNO(3)to,(p)"One of the products...
Text Solution
|
- {:("Column I","Column II"),((A)2NO(2)overset("Cool")to,(p)"One of the ...
Text Solution
|
- P(4) reduces copper sulphate solution to metallic copper.
Text Solution
|
- Red phosphorus catches fire at room tempt.
Text Solution
|
- Hyderoxylamine undergoes disproportination reaction rapidly in alkalin...
Text Solution
|
- N(2)O does not from hyperitrite with alkali.
Text Solution
|
- In alkaline solution, with Devarda's alloy (Cu//Al//Zn) nitrites are r...
Text Solution
|
- N(2)O is an acid anhydride of HNO(3).
Text Solution
|
- SO(3) does not turn starch iodate paper blue.
Text Solution
|
- Reduction of bisulphite solution and SO(2) with zin dust yields sulpha...
Text Solution
|