Text Solution
Verified by Experts
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PRADEEP|Exercise NCERT (Questions and exercise )( NCERT EXERCISES)|40 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PRADEEP|Exercise NCERT (EXEMPLAR PROBLEMS) (Multiple Choice Questions -I)|13 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PRADEEP|Exercise Conceptual Questions|10 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise Curiosity Questions|2 VideosENVIRONMENTAL CHEMISTRY
PRADEEP|Exercise COMPETITION FOCUS (JEE(Main and Advanced)/Medical Entrance (VI.ASSERTION-REASON) Type II|6 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES -NCERT (Questions and exercise )(NCERT INTEXT SOLVED QUESTIONS)
- What would be the IUPAC name and symbol for the element with atomic nu...
Text Solution
|
- How would you justify the presence of 18 elements in the 5th period of...
Text Solution
|
- The elements Z = 117 and 120 have not yet have been discovered, In whi...
Text Solution
|
- Considering the atomic number & position in the periodic table, arrang...
Text Solution
|
- Which of the following species will have the largest and the smallest ...
Text Solution
|
- The first ionisation enthalpy (Delta(i)H^(o-)) values of the third per...
Text Solution
|
- Which of the following will have the most negative electron gain...
Text Solution
|
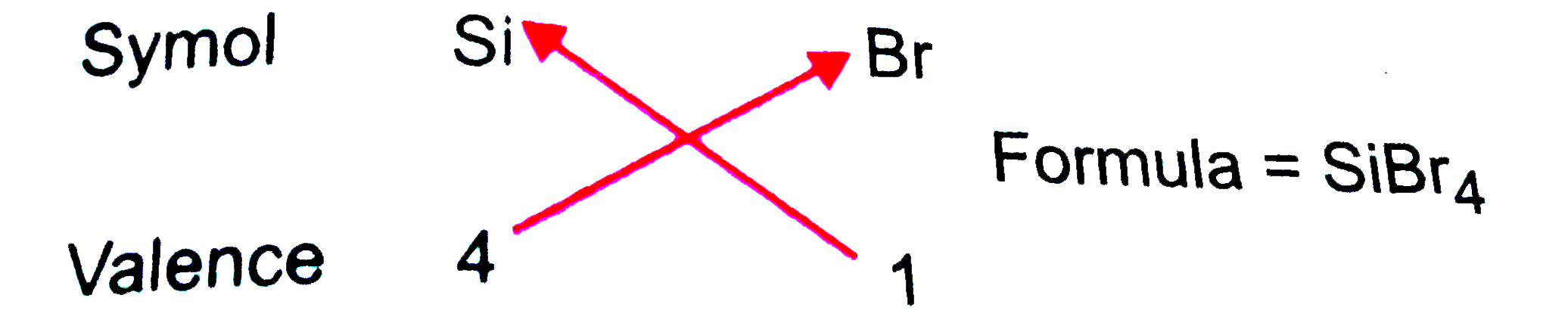
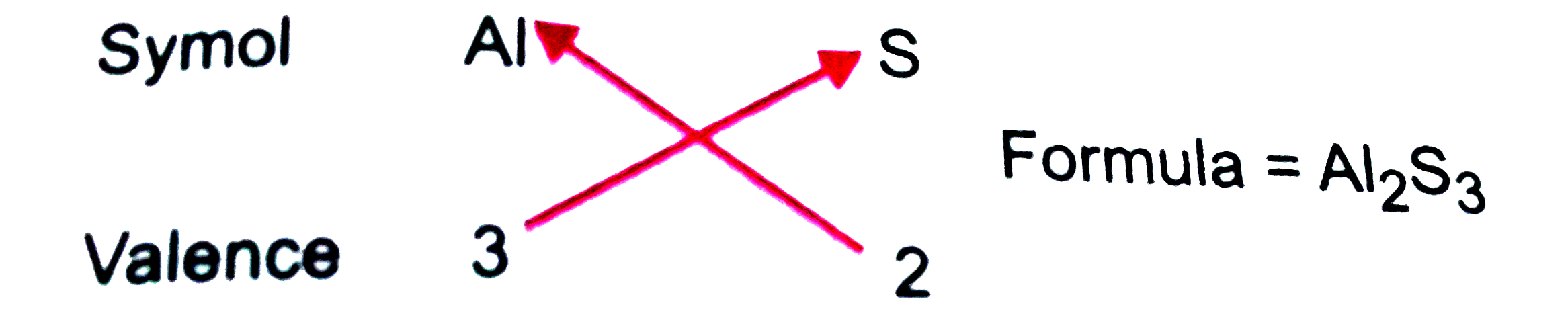
- Using the perodic table, perdict the formulas of compounds which migh...
Text Solution
|
- Are the oxidation state and covalency of Al in [AlCl(H(2)O)(5)]^(2+) s...
Text Solution
|
- Show by a chemical reaction with water that Na(2)O is a basic oxide an...
Text Solution
|