Five millilitires of a gas (A) containing only C and H was mixed with an excess of oxygen (30 ml) and the mixture was exploded by means of an electric spaek. After the explosion, the remaining volume of the mixed gasses was 25 ml. On adding a concentrated solution of KOH, the volume further diminished to 15 ml. The residual gas being pure oxyges.
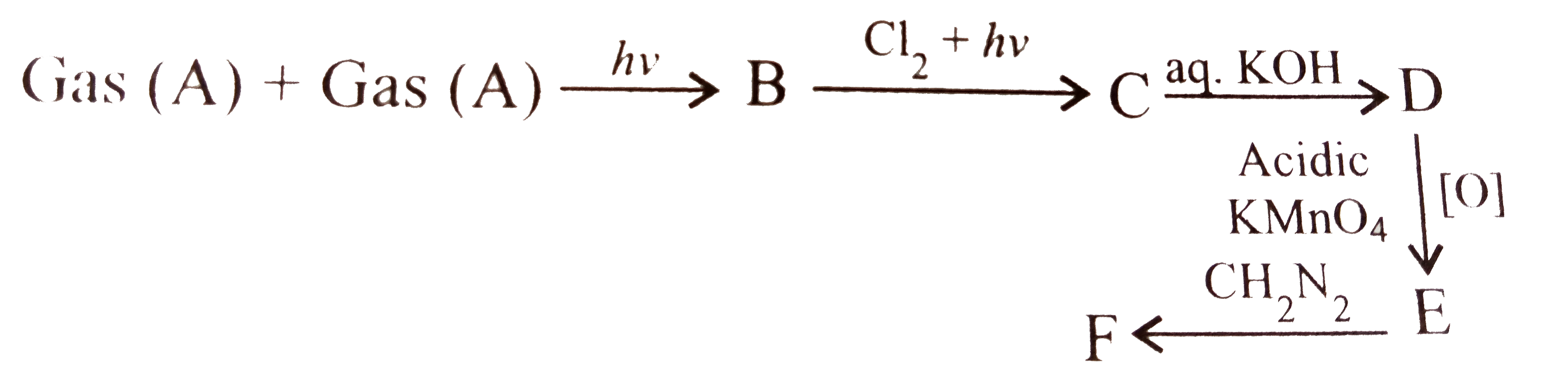
The molecular formula of gas (A) is: