Mass of chlorin in `1.0 g X = (35.5)/(143.5) xx 2.9 = 0.717 g`
Now, the empirical formula can be derived as :
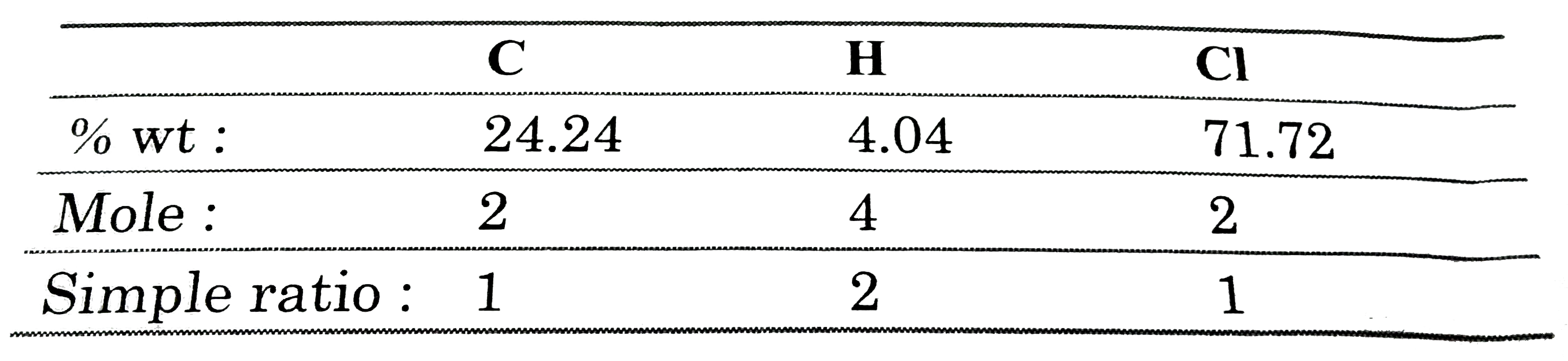
`rArr` Empirical formula `= CH_(2)Cl`.
Because X canbe represented by two formula of which one gives a dihydroxy compound with `KOH` indicates that x has two chlorine atoms per molecules.
`rArr X = C_(2)H_(4)Cl_(2)` with two of its structural isomers.
`underset(I)(Cl-CH_(2)-CH_(2)-Cl)` and `underset(II)(CH_(3)-CHCl_(2))`
On treatment with `KOH, I` will give ethane -1,2-diol, hence it is Y, Z one treatment with `KOH` will give ethanal as
`ClCH_(2)CH_(2)Cl+OH^(-)rarr underset((Y))(underset(OH)underset(|)(CH_(2))-underset(OH)underset(|)(CH_(2)))`
`CH_(3)CHCl_(2)+KOH rarr underset("Unstable")(CH_(3)CH(OH)_(2))overset(-H_(2)O)rarrunderset((Z))(CH_(3)CHO)`