`4.0 g` of a mixture of `NaCl` and an unknown metal iodide `MI_(2)` was dissolved in water to form its aqueous solution. To this aqueous solution, aqueous solution of `AgNO_(3)` was added gradually so that silver halides are precipitated. The precipitates were weighed at regular interval and following curve for the mass of precipitate versus volume of `AgNO_(3)` added was obtained. With the knowledge of the fact that halides are precipitated successively, i.e, when less soluble halide is precipitating, the other halide remain in the solution, answer the following questions: (Molar mass of `Ag = 108, I = 127, Na=23`).
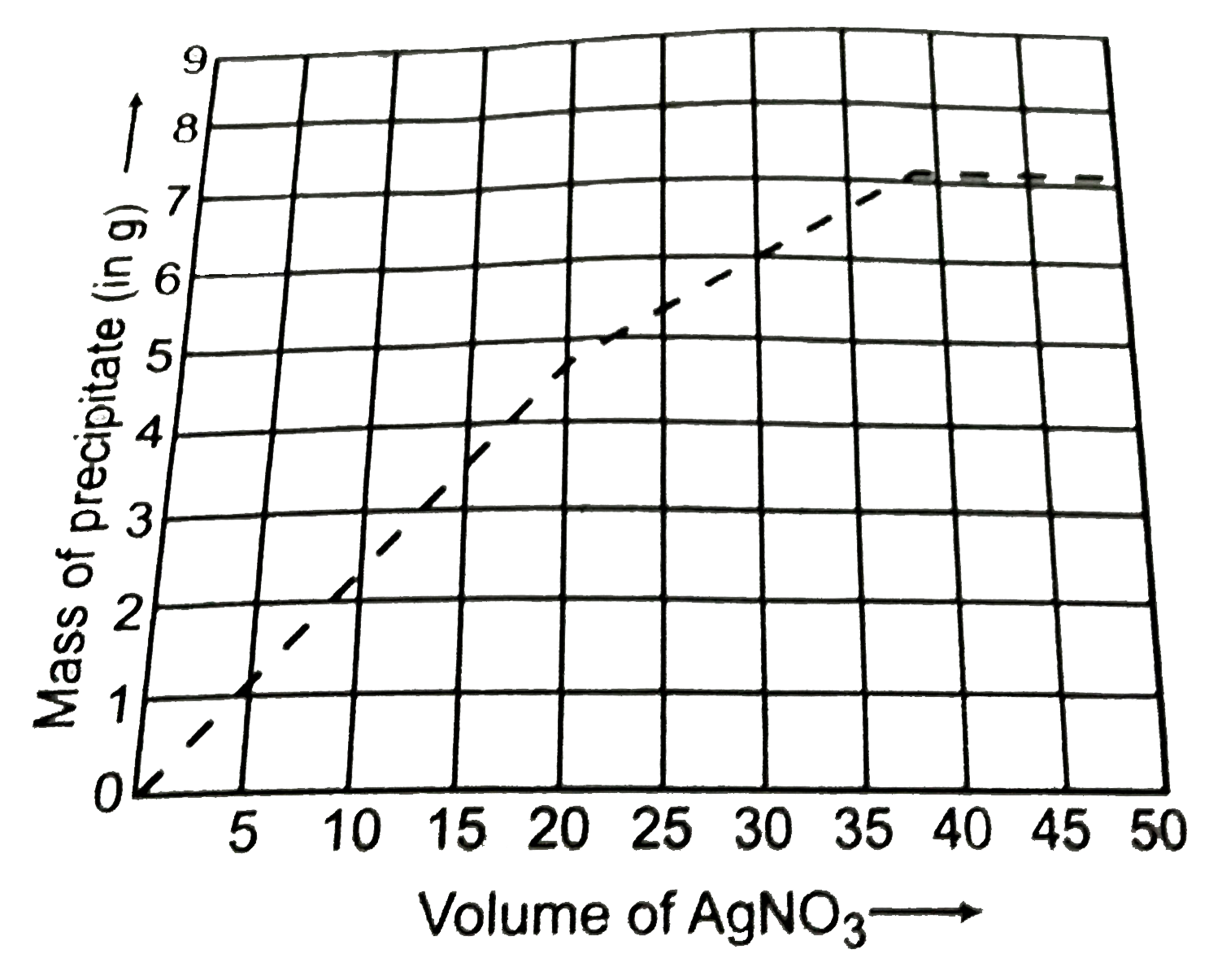
What is the approximate molar mass of unknown metal M?