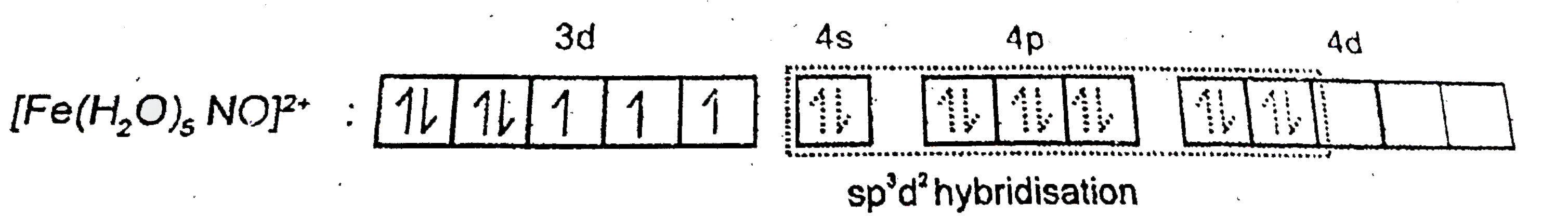A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-TEST PAPERS-Pt-05
- Which of the following statements is not correct ?
Text Solution
|
- A metal salt solution gives raddish brown precipitate with ammonium hy...
Text Solution
|
- Reaction of hydrated ferric chloride (FeCl(3).6H(2)O) with thionyl chl...
Text Solution
|
- How many isomers of the complex, Pt(NH(3))(2)(SCN)(2) are possible
Text Solution
|
- Match the complexed listed column -I with type of hybridisation liste...
Text Solution
|
- Match the properties given in Column I with the reansition elements gi...
Text Solution
|
- Match coloumn I with column II and select the correct answer using the...
Text Solution
|
- The total number of carbonate ores among the following is . ltBRgt (i)...
Text Solution
|
- A greenish yellow gas reacts with an alkin metal hydroxide to form a h...
Text Solution
|
- Single N-N bond is weaker than the single P-P bond. This is because ...
Text Solution
|
- Identify the correct order of wavelength of light absorbed for the fo...
Text Solution
|
- Which of the following orders is not correct with respects to the prop...
Text Solution
|
- What are the products formed in the reaction of xenon hexafluoride wit...
Text Solution
|
- Match the complexes given in column -I and the magnetic properties giv...
Text Solution
|
- underset((X))(K(6)[(CN)(5)" "Co-O-O-Co(CN)(5)])overset("oxidizes")rarr...
Text Solution
|
- Consider the following statements and select the correct option using ...
Text Solution
|
- Match the method of concentration of the ore in column I with the ore ...
Text Solution
|
- Consider the following statements : S(1) : In extration of iron from...
Text Solution
|
- Which one of the following properities is correct for ozone ?
Text Solution
|
- The manufacture of fluorine is done by
Text Solution
|