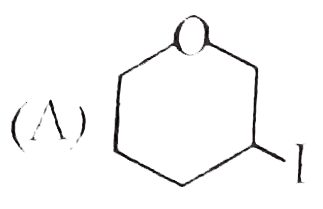
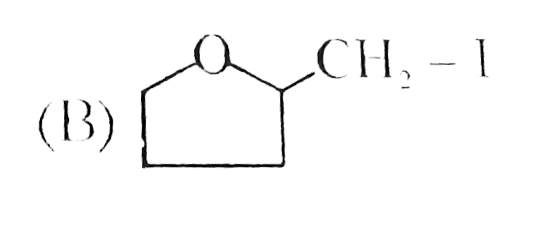
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-TEST PAPERS-part-2 chemistry
- The most important copper oxide containing minerals are azurite {Cu(3)...
Text Solution
|
- Alkenes undergo electrophilic addition reactions. Electrophilic additi...
Text Solution
|
- Alkenes undergo electrophilic addition reactions. Electrophilic additi...
Text Solution
|
- Alkenes undergo electrophilic addition reactions. Electrophilic additi...
Text Solution
|
- Match the pairs of compounds (to be distinguished) given in Column-(I)...
Text Solution
|
- {:("Coloumn-I",,"Column-II"),("(Aqueous solution in standard condition...
Text Solution
|
- Roasting is carried out in extraction of metal in how many ores/minera...
Text Solution
|
- Calculate number of significant digits in Avogadro number if Boltzmann...
Text Solution
|
- We have taken a saturated solution of AgBr, whose K(sp) is 12xx10^(-14...
Text Solution
|
- 2 moles of KMnO(4) can oxidise x moles of hydrogen peroxide in weakly ...
Text Solution
|
- Select the correct statement for a real gas (i)The behaviour of the ...
Text Solution
|
- Select the incorrect statement
Text Solution
|
- Two stereoisomers (Shown in fischer projection ) from an epoxide, when...
Text Solution
|
- Match each of the following four structures with one of the compound A...
Text Solution
|
- A white crystalline solid (A) on boiling with caustic soda solution ga...
Text Solution
|
- A reagent is added to a solution of manganous salt in cold dil. HNO(3...
Text Solution
|
- Defects are basically irregularities in the arrangement of constituent...
Text Solution
|
- Defects are basically irregularities in the arrangement of constituent...
Text Solution
|
- Compound (A) has molecular formula C(5)H(12)O. It liberates H(2) gas o...
Text Solution
|
- compound (A) forms a ketone. It undergoes in following reaction scheme...
Text Solution
|