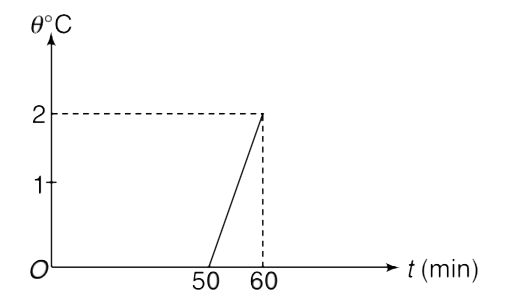
A container contains 5 kg of water at `0^(@)C` mixed to an unknown mass of ice in thermal equilibrium. The water equivalent of the container is 100 g. At time t = 0, a heater is switched on which supplies heat at a constant rate to the container. The temperature of the mixture is measured at various times and the result has been plotted in the given figure. Neglect any heat loss from the mixture – container system to the surrounding and calculate the initial mass of the ice.
Given: `S_(p)`. latent heat of fusion of ice is `L_(f) = 80 cal g^(-1)`
`S_(p)`. heat capacity of water `= 1 cal g^(-1) .^(@)C^(-1)`