100 g of ice at `-40^(@)C` is supplied heat using a heater. The heater is switched on at time t = 0 and its power increases linearly for first 60 second and thereafter it becomes constant as shown in the graph. Heater is kept on for 5 minutes. The specific heat capacity for ice and water are known to be `2.1 (J)/(g^(@)C)` and `4.2 (J)/(g^(@)C)` respectively. The specific latent heat for fusion of ice is `336 J//g`.
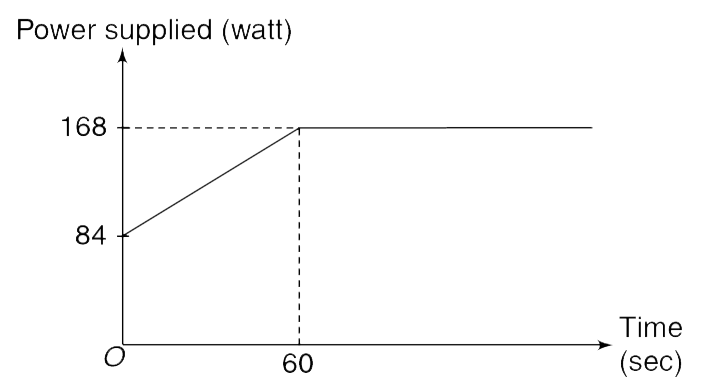
The temperature of the ice sample kept on increasing till time `t_(1)` and then remained constant in the interval `t_(1) lt t lt t_(2)`.
(i) Find `t_(1)` and `t_(2)`
(ii) Find final temperature of the sample when the heater is switched off.