Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ARIHANT-CALORIMETRY-Level 2
- A container has a square cross-section of 10 cm xx 10 cm. A cubical ic...
Text Solution
|
- Two identical cylindrical containers A and B are interconnected by a t...
Text Solution
|
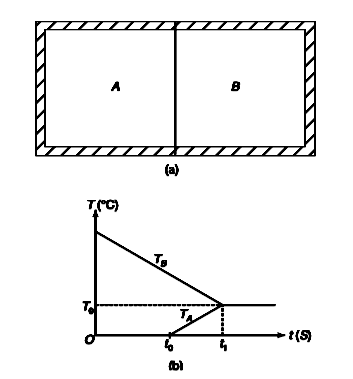
- A well insulated box has two compartments A and B with a conducting wa...
Text Solution
|
- An ice ball has a metal piece embedded into it. The temperature of the...
Text Solution
|
- Water from a reservoir maintained at a constant temperature of 80^(@)C...
Text Solution
|
- A cylindrical container has a cross sectional area of A(0) = 1 cm^(2) ...
Text Solution
|