Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ARIHANT-FIRST LAW OF THERMODYNAMICS-Level 2
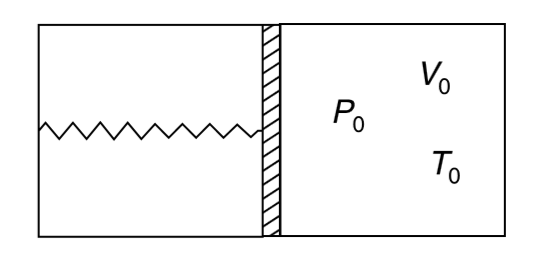
- A container has a tight fitting movable piston which can slide without...
Text Solution
|
- One mole of a gas is in state A[P, V, T(A)]. A small adiabatic process...
Text Solution
|
- A metal cylinder of density d, cross sectional area A and height h is ...
Text Solution
|
- n moles of an ideal mono atomic gas is initially at pressure 32 P(0) a...
Text Solution
|
- One end of an insulating U tube is sealed using insulating material. A...
Text Solution
|
- Air is filled inside a jar which has a pressure gauge connected to it....
Text Solution
|
- The molar specific heat capacity at constant volume (C(V)) for an idea...
Text Solution
|
- A sample of oxygen is heated in a process for which the molar specific...
Text Solution
|
- A gas-gun has a cylindrical bore made in an insulating material. Lengt...
Text Solution
|
- During a process carried on an ideal gas it was found that eta (lt 1) ...
Text Solution
|
- An ideal gas is taken through a thermodynamic cycle ABCDA. In state A ...
Text Solution
|
- (i) A conducting piston divides a closed thermally insulated cylinder ...
Text Solution
|
- A spherical container made of non conducting wall has a small orifice ...
Text Solution
|
- A glass tube is inverted and dipped in mercury as shown. One mole of a...
Text Solution
|
- Figure shows P versus V graph for various processes performed by an id...
Text Solution
|
- n' moles of an ideal gas having molar mass M is made to undergo a cycl...
Text Solution
|
- In the arrangement shown in figure, the piston can smoothly move insid...
Text Solution
|
- There is a long vertical tube of radius r containing air at atmospheri...
Text Solution
|
- A ball of radius r fits tightly inside a tube attached to a container....
Text Solution
|
- An ideal mono atomic gas is at temperature T(0). The pressure and volu...
Text Solution
|