Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
IIT-JEE PREVIOUS YEAR (CHEMISTRY)-EXTRACTION OF METALS-JEE Main And Advanced
- The number of water molecule(s) derectly bonded to the metal centre in...
Text Solution
|
- Write the balanced chemical equations for developing a black and white...
Text Solution
|
- A(1) and A(2) are two ores of metal M.A(1) on calcination gives a blac...
Text Solution
|
- Which one is more soluble in diethyl ether : anhydrous AlCl(3) or hydr...
Text Solution
|
- Write down the reactions involved in the extraction of Pb. What of the...
Text Solution
|
- Write the balanced chemical equation for developing photographic films...
Text Solution
|
- Write the chemical reactions involved in the extraction of metallic si...
Text Solution
|
- In moist air, copper corrodes to produce a green layer on the surface....
Text Solution
|
- When the ore haematite is burnt in air with coke around 2000 K along w...
Text Solution
|
- Give balance equation for the reaction of aluminium with aqueous sodiu...
Text Solution
|
- Write a balanced equation for the reaction of argentite with KCN and n...
Text Solution
|
- Give reasons for the following. "Although aluminium is above hydrogen ...
Text Solution
|
- Complete the following reaction: Sn+2KOH+4H(2)Orarr……+…….
Text Solution
|
- Give briefly the isolation of magnesium from sea water. Give equation...
Text Solution
|
- Calculate the number of moles of Cu and HNO(3) to give NO and NO(2) in...
Text Solution
|
- Write balanced equations for "the extraction of copper from pyrites by...
Text Solution
|
- Give balanced equations for the following. "Extraction of silver fro...
Text Solution
|
- Answer the following questions briefly. (i) What is the actual reduc...
Text Solution
|
- Write the balanced equations for the reaction occuring when gold is di...
Text Solution
|
- Each of the following statements is true only under some specific cond...
Text Solution
|
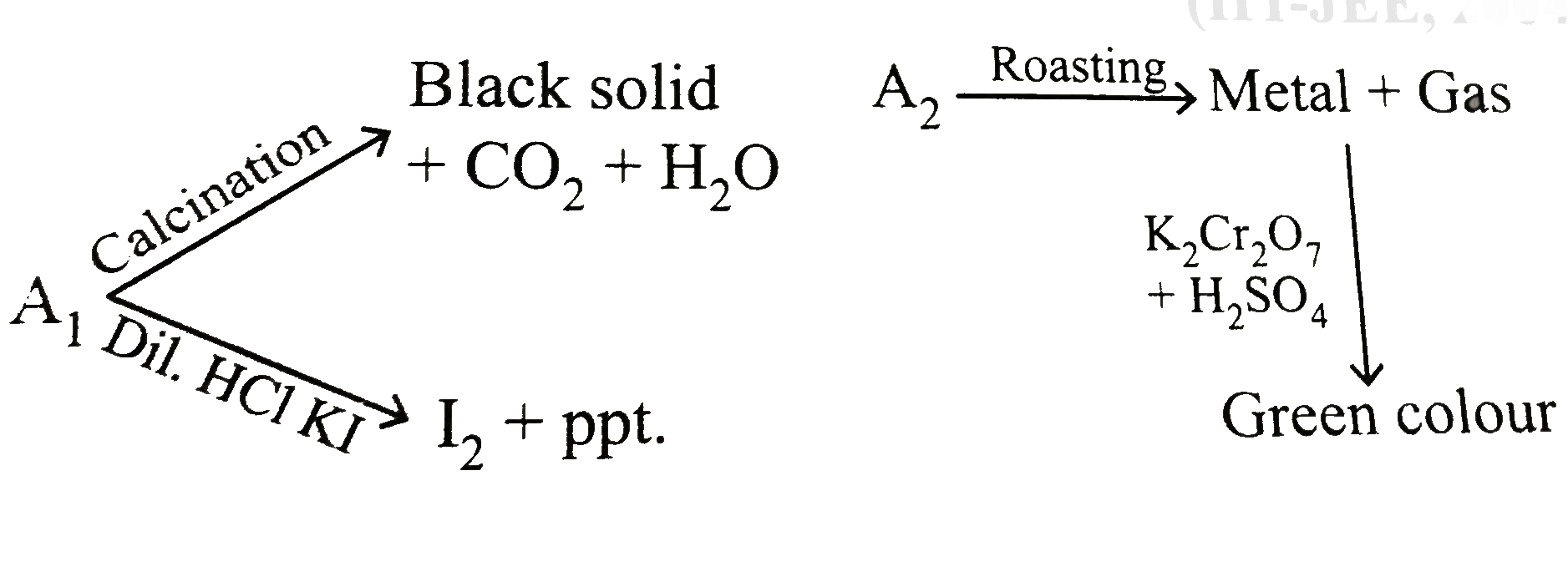 .
.