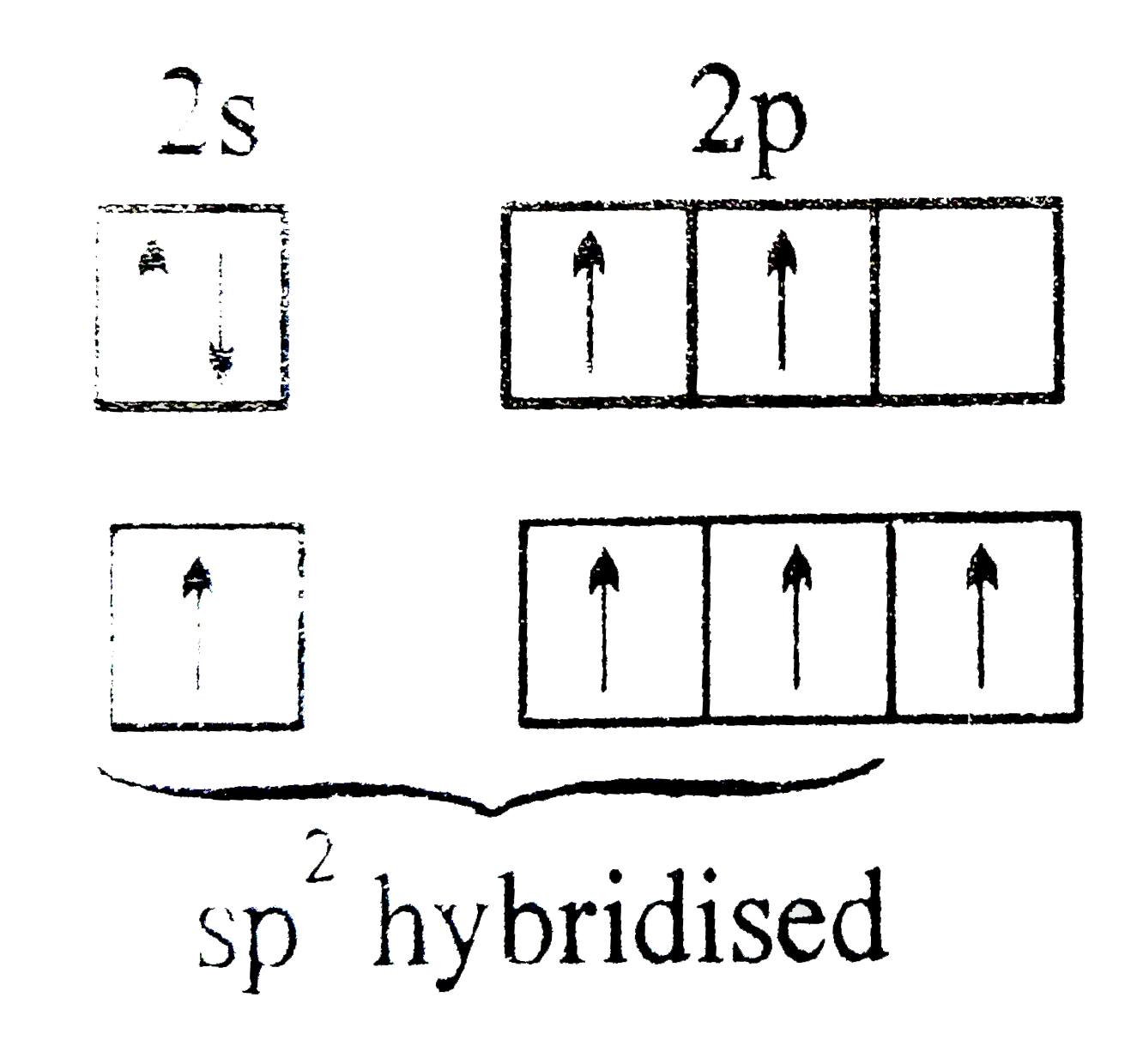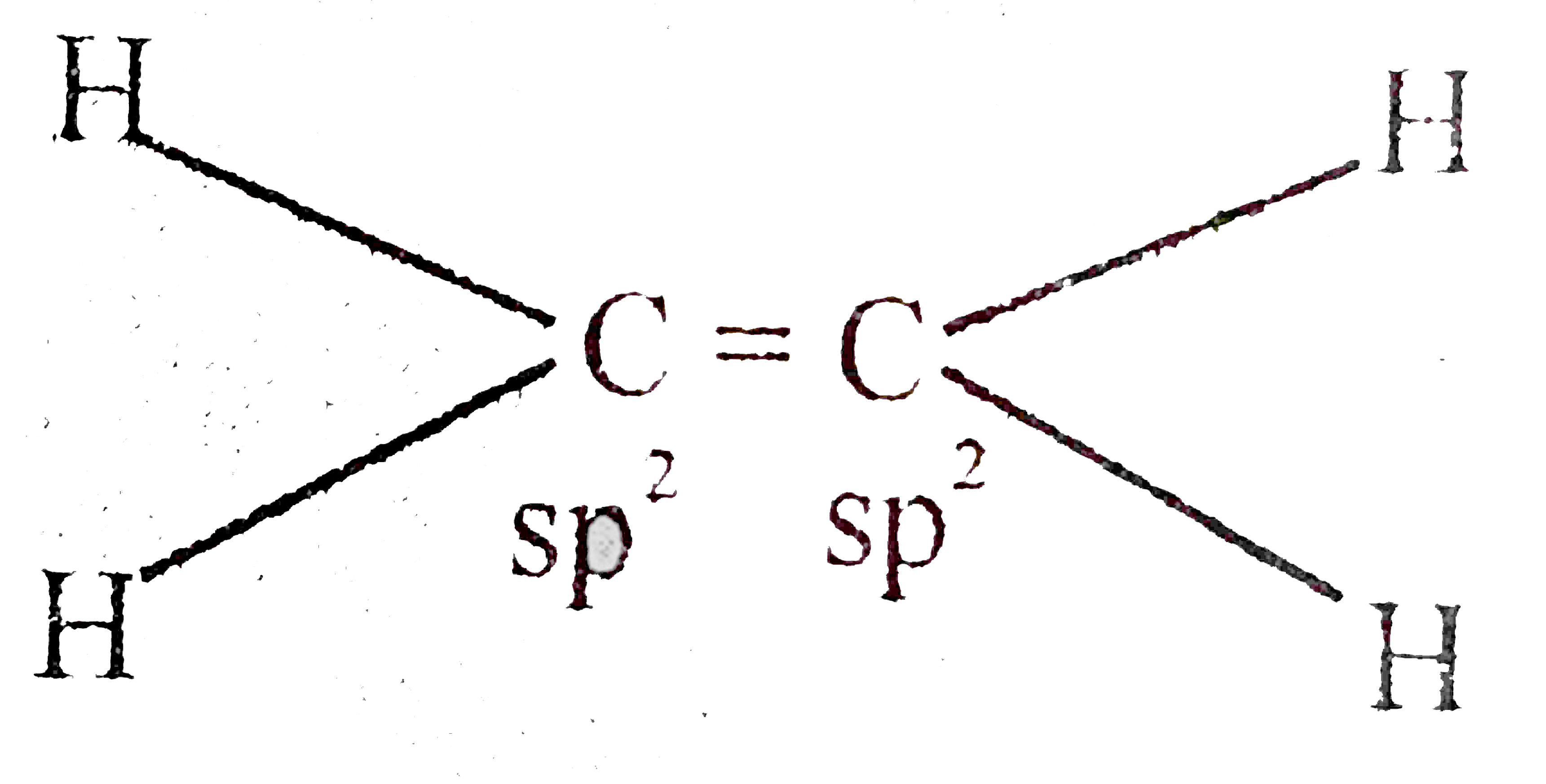A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-TEST PAPERS-CHEMISTRY
- Choose the correct order from the following :
Text Solution
|
- Accroding to Lewis Octet structure, which is correct for SO(4)^(2-)
Text Solution
|
- Ethylene molecules is formed as a result of sp^(2) hybridisation of ca...
Text Solution
|
- Ethylene molecules is formed as a result of sp^(2) hybridisation of ca...
Text Solution
|
- Ethylene molecules is formed as a result of sp^(2) hybridisation of ca...
Text Solution
|
- Select the pair which follows energy order according to (n+l) rule (Au...
Text Solution
|
- Which of the following sub-shell does not exist for an atom, according...
Text Solution
|
- Which of the following has sp^(3)d^(2) hybridisation ?
Text Solution
|
- In which of the following d(z^(2)) orbital is participating in hybridi...
Text Solution
|
- {:("Column-I",,"Column-II"),("(A) Electron affinity",,"(P) Depends u...
Text Solution
|
- Calculate the sum of simga and pi bond in tetra phosphoric acid. ...
Text Solution
|
- The number of d-orbital participating in hybridisation in IF(7) are .
Text Solution
|
- How many molecules have two lone pairs on the central atom ? H(2)O,...
Text Solution
|
- For atom 'A' ionisation energy is given in eV. {:(I.E.(1),I.E.(2),I...
Text Solution
|
- Which of the following molecules or species has different bond angles ...
Text Solution
|
- In which of the following O-N-O bond angles is highest ?
Text Solution
|
- Find the species having highest value of magnetic moment in their gro...
Text Solution
|
- Which of the following option consist of substances that will illustra...
Text Solution
|
- Select the order of ionic radii :
Text Solution
|
- Identify the option containing maximum number of atoms
Text Solution
|