Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-TEST PAPERS-CHEMISTRY
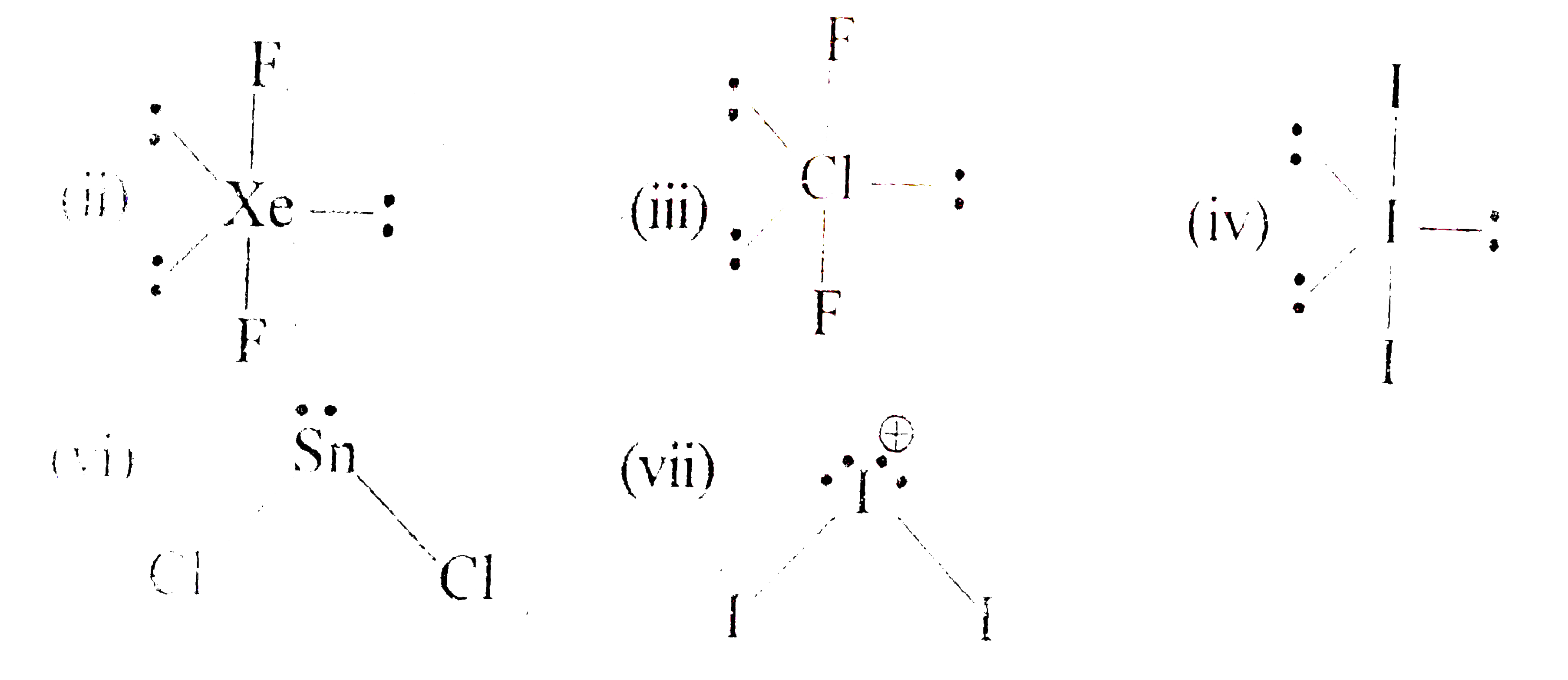
- Match parameters involved in column I with those in column II. {:("C...
Text Solution
|
- How many molecules or ions have minimum tow lone pairs on the centre a...
Text Solution
|
- How many molecules or ions are linear in shape ? {:(BeCl(2),XeF(2),...
Text Solution
|
- Find the number of species where d(x^(2)-y^(2)) orbital participate in...
Text Solution
|
- Calculate total number of SO(2) molcules in a sample having 32 miligra...
Text Solution
|
- Which of the following option is incorrect
Text Solution
|
- Compound having least lattice energy is
Text Solution
|
- which of the following options correctly represent mass of 10 molecule...
Text Solution
|
- Identify in which of the following case can the average molecular mass...
Text Solution
|
- Which of the following elemts will have the same total number of elect...
Text Solution
|
- In which of the following set of molecules central atoms are sp^(3) hy...
Text Solution
|
- The density of a pure liquid (molecular mass =80) is 1.5 gm/ml. if 4 m...
Text Solution
|
- Two different formulas are used in order to represent composition of a...
Text Solution
|
- Two different formulas are used in order to represent composition of a...
Text Solution
|
- Two different formulas are used in order to represent composition of a...
Text Solution
|
- Which of the following has zero dipole moment value ?
Text Solution
|
- A particular elements 'X' can be found in three gaseous forms -mono-at...
Text Solution
|
- A sample of oxygen atoms contain only .(8)O^(16) and .(8)O^(18) isotop...
Text Solution
|
- {:("Column-I",,"Column-II"),("(A) " BF(4)^(-),,"(P) All atoms are p-bl...
Text Solution
|
- Find the number of compounds which do not exist. {:(ClF(3),BrF(5),H...
Text Solution
|