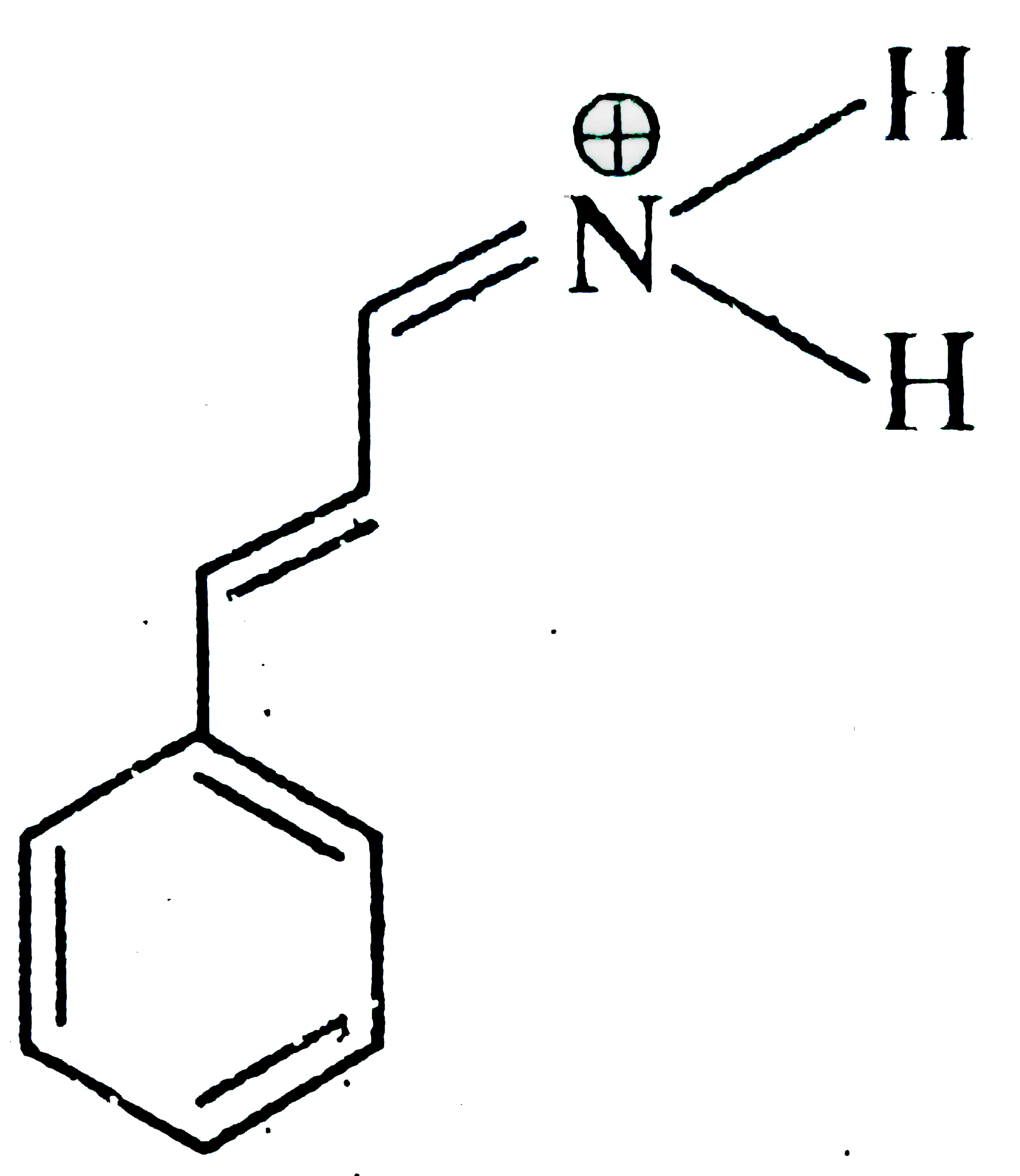
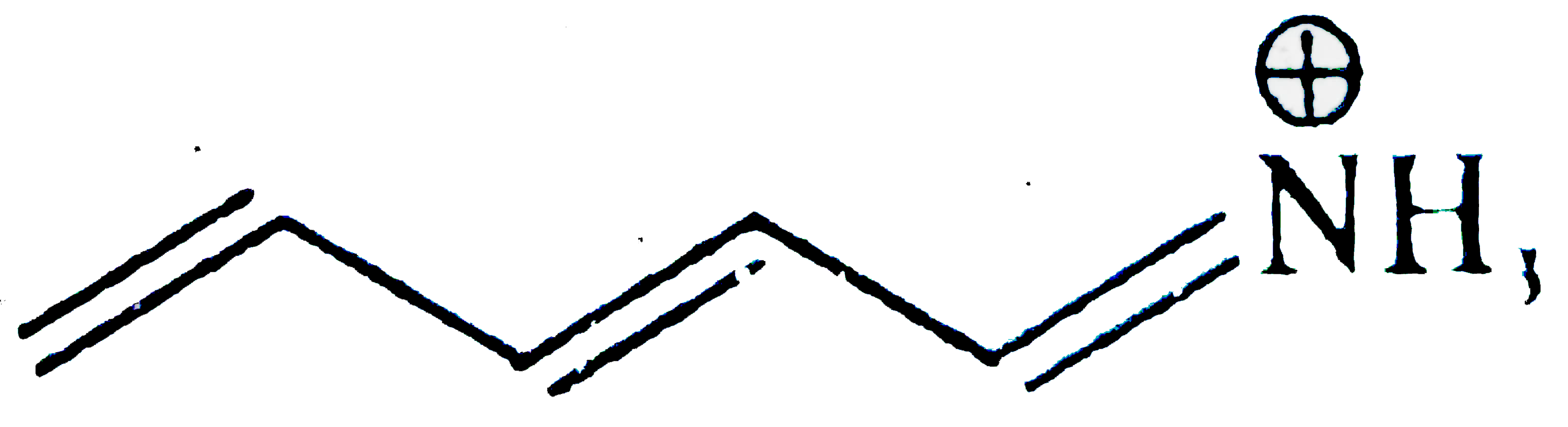
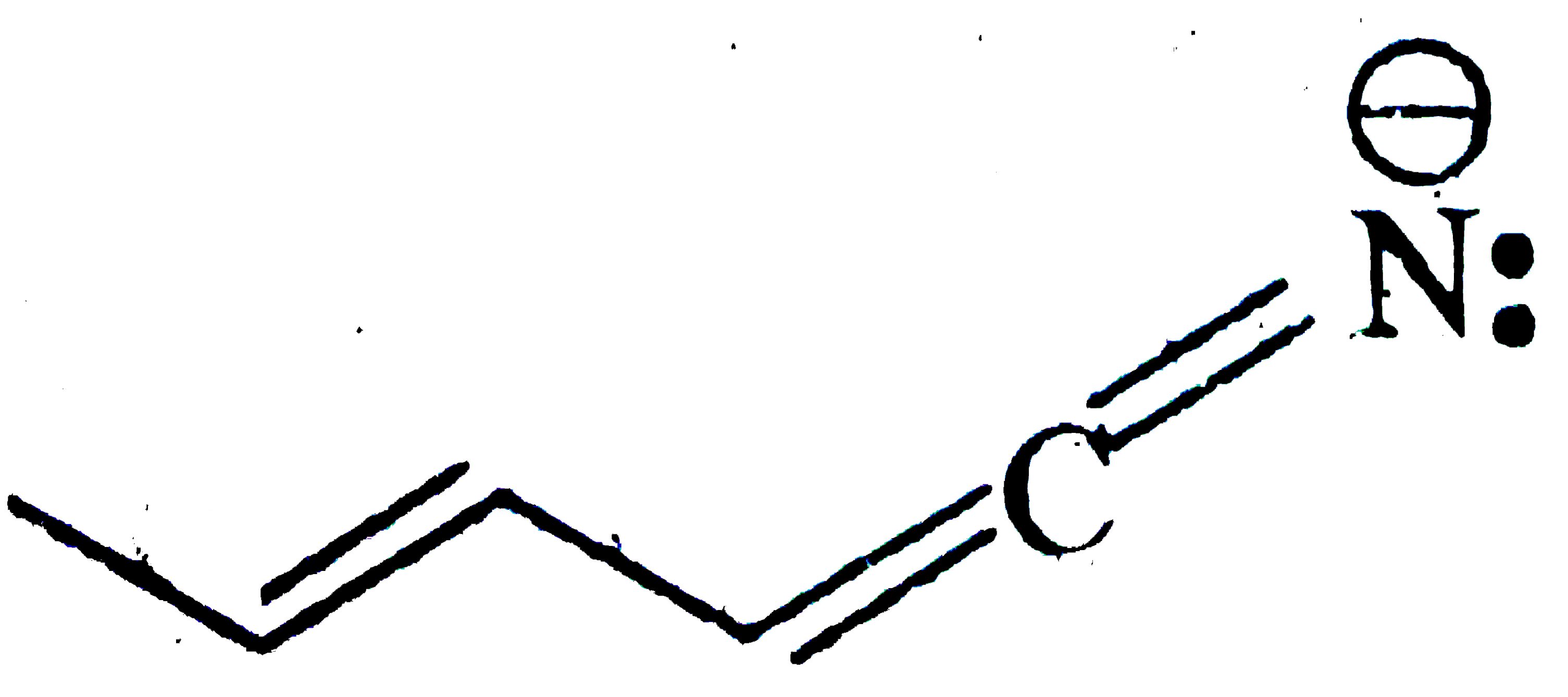
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-TEST PAPERS-CHEMISTRY
- An alpha- particle accelerated through V volt is fired towards a nucle...
Text Solution
|
- Which of the following ions/compounds does not exist?
Text Solution
|
- Which of the following is not a valid resonating structure?
Text Solution
|
- When x gm carbon is burnt with y gm oxygen in a closed vessel, no resi...
Text Solution
|
- Hydration energy of the given ions follows the order.
Text Solution
|
- In the following there are three carbon-oxygen bonds denoted by x, y a...
Text Solution
|
- Photoelectric emission is observed from a surface for frequencies v1 a...
Text Solution
|
- Which of the following can form intermolecular H-bonding between its m...
Text Solution
|
- When the compounds CH3COOH, C2H5OH and C6H5OH are arranged in order of...
Text Solution
|
- A hydrogen like species with atomic number Z is present in a higher ex...
Text Solution
|
- Which of the following is the most acidic in nature?
Text Solution
|
- IUPAC name of this compound is :
Text Solution
|
- The time period of revolution in the 3^(rd) orbit of Li^(2+) ion is x ...
Text Solution
|
- Which of the following is not a Homocyclic compound.
Text Solution
|
- A potential difference of 30KV is applied across an X-ray tube. Find t...
Text Solution
|
- Select compound having maximum solubility in water.
Text Solution
|
- Following graphs are obtained when different gases are subjected to ch...
Text Solution
|
- Weakest base among the following is:
Text Solution
|
- 0.1 mole of argon has pressure P & temperature TK in the vessel. On ke...
Text Solution
|
- Which of the following is strongest base?
Text Solution
|