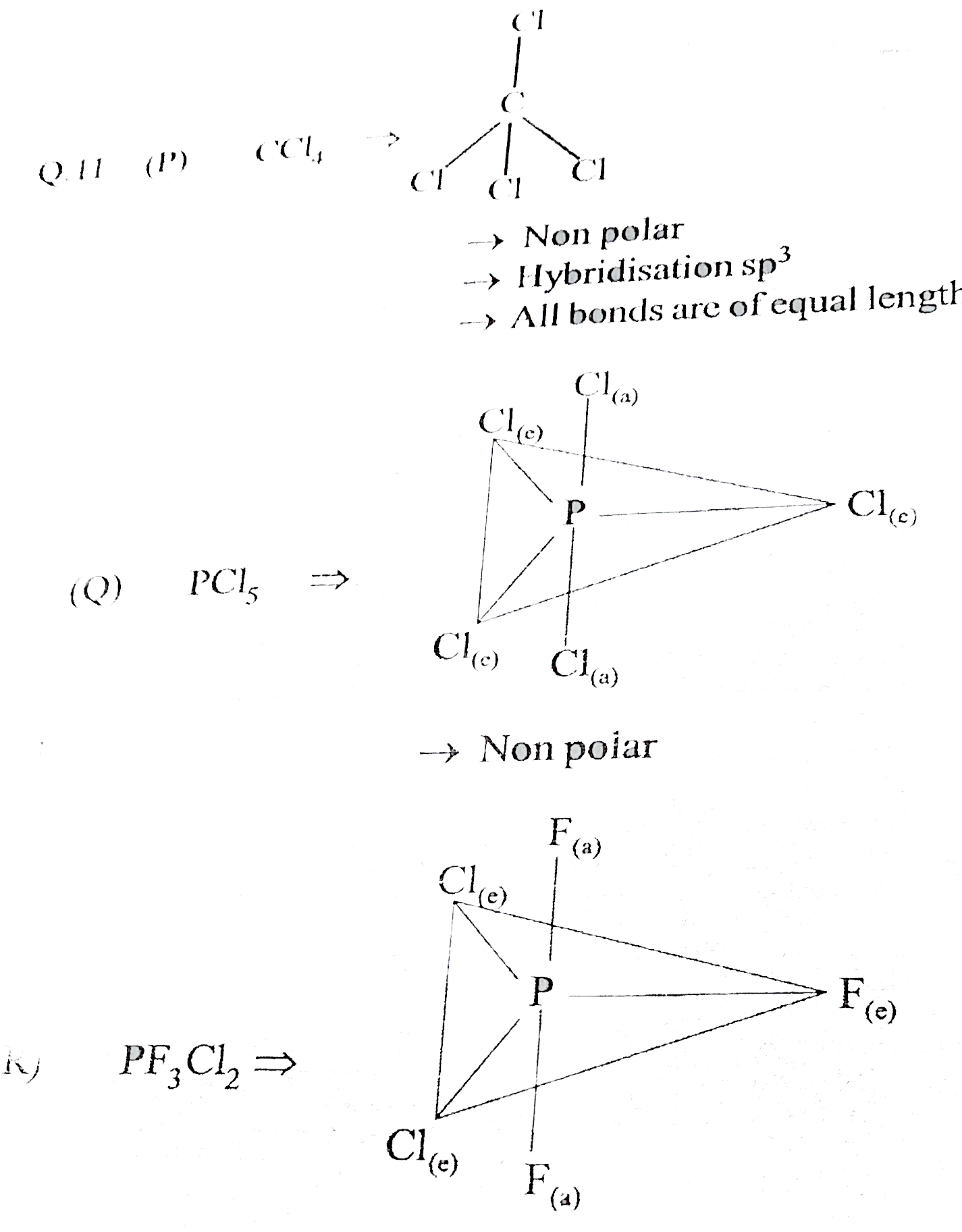A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-TEST PAPERS-CHEMISTRY
Text Solution
|
- 6 litre H(2)O is placed in a closed evacuted room of volume 8.27 litre...
Text Solution
|
- {:("List I",,"List II"),("(P) CC " l(4),,"(1) maximum 3 bonds are of e...
Text Solution
|
- Match list-I with list -II and select the correct answer. {:("List I...
Text Solution
|
- Select compound having maximum solubility in water.
Text Solution
|
- Which of the following compounds act as Lewis acid as well as Lewis ba...
Text Solution
|
- Which compounds are more basic than
Text Solution
|
- Calculate pressure (in atm) of a Vander-waal gas taken at temperature ...
Text Solution
|
- Find the total number of P-S-P linkages in P(4)S(10) ?
Text Solution
|
- Consider given compounds & write the number of compounds which have hi...
Text Solution
|
- According to Maxwell's theory of electron dynamics, an electron going ...
Text Solution
|
- How many compounds are chain, positional or functional group isomer of...
Text Solution
|
- An excited hydrogen atom emits a photon of wavelength lambda in return...
Text Solution
|
- Which of the following has monoatomic anion in the solid state?
Text Solution
|
- Indentify strongest acid amongst followings.
Text Solution
|
- 10 ml of ethane gas is mixed with 40ml oxygen gas in an eudiometer tub...
Text Solution
|
- The correct order of thermal stability is
Text Solution
|
- What is correct IUPAC name of
Text Solution
|
- As per KTG, molecules in gaseous state are under continuous motion wit...
Text Solution
|
- As per KTG, molecules in gaseous state are under continuous motion wit...
Text Solution
|