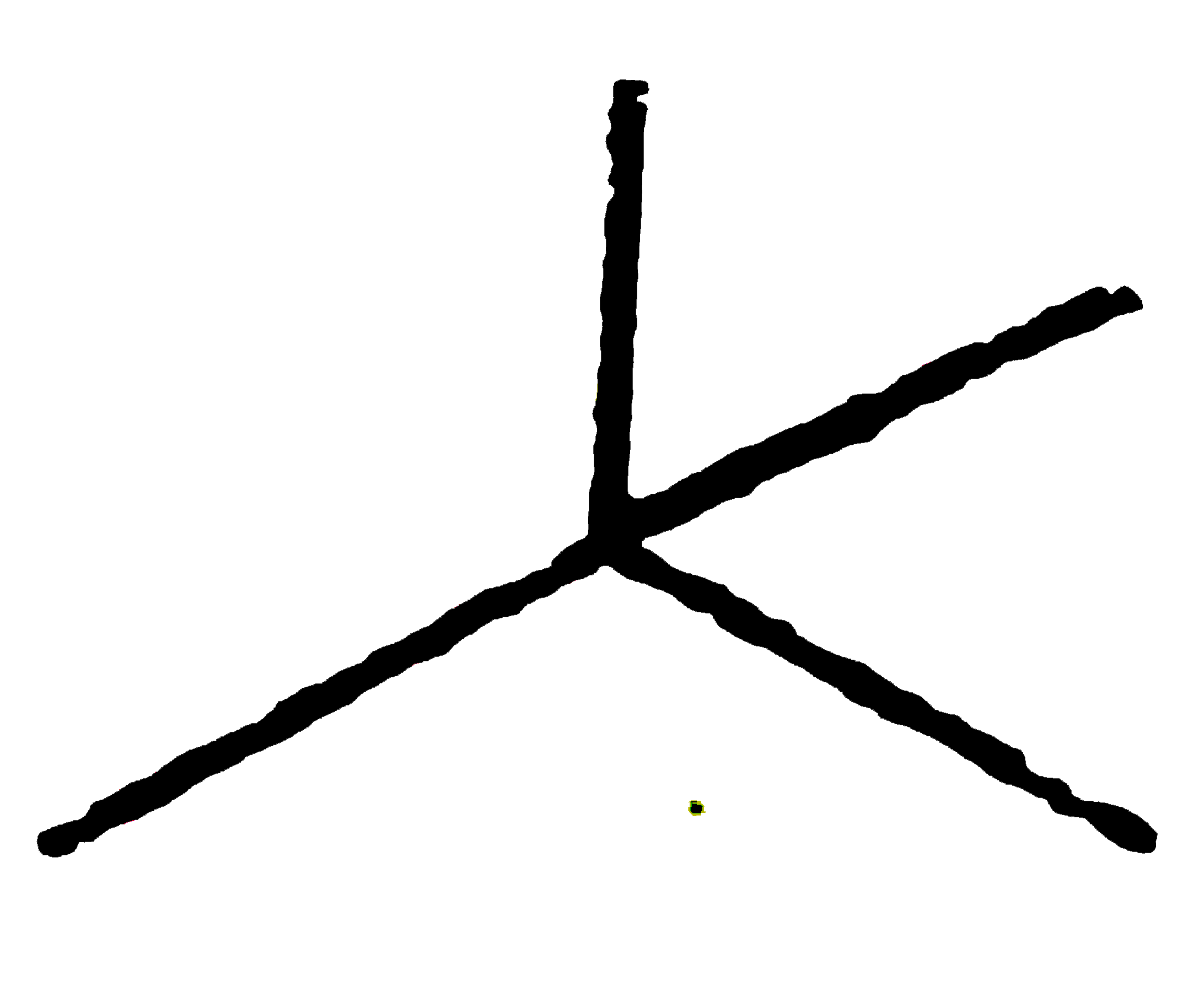
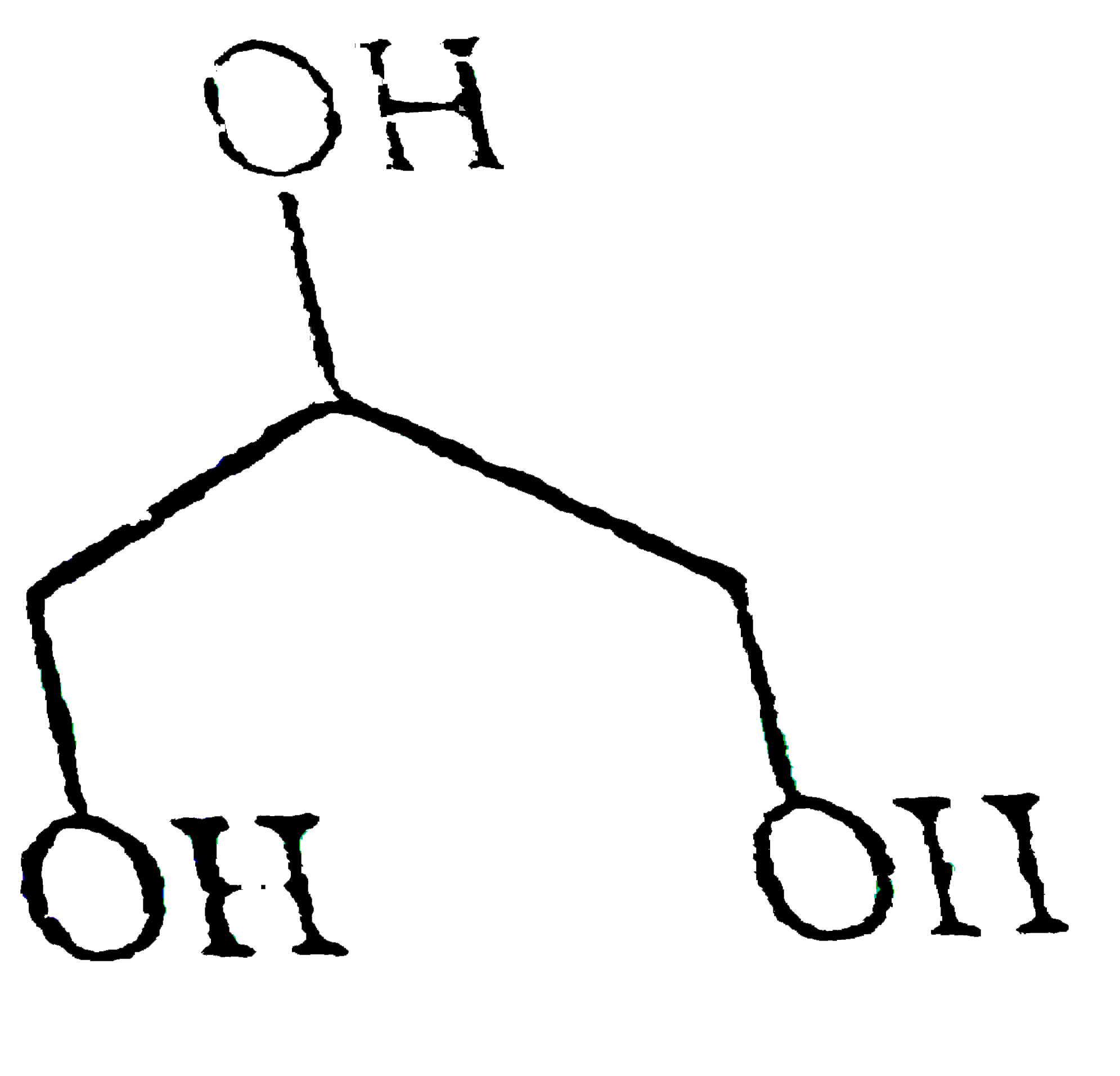A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-TEST PAPERS-CHEMISTRY
- As per KTG, molecules in gaseous state are under continuous motion wit...
Text Solution
|
- When photons of energy 4.25eV strike the surface of a metal A, the eje...
Text Solution
|
- Which compounds have high boiling point in comparision to
Text Solution
|
- Select the correct statement regarding XeF2
Text Solution
|
- Which option(s) is/are incorrect.
Text Solution
|
- {:("Column I",,"Column II"),("(A)" overset(Theta)(C)H(3),,"(P) Nucleop...
Text Solution
|
- Calculate an integer obtained by adding solution codes of all true sta...
Text Solution
|
- Amongst given compound, number of compounds which can evolve NH3 on re...
Text Solution
|
- An unspecified quantity of an ideal gas was at initial pressure of 5 a...
Text Solution
|
- Consider following compounds & give number of compounds which have HOH...
Text Solution
|
- If an electron has spin quantum number value is + (1)/(2) and magnetic...
Text Solution
|
- The numerical value of energy involved in the given process , S rarr S...
Text Solution
|
- Element which has maximum ionisation energy
Text Solution
|
- Which of the following has least first electron affinity ?
Text Solution
|
- Which of the following pair of element has incorrect order of atomic r...
Text Solution
|
- Statement-1 : The ground state configuration of Cr is [Ar] 3d^(5) 4s^(...
Text Solution
|
- Identify the group number of T hat(l) element in periodic table.
Text Solution
|
- If Hund Rule is violate, then which of the following species is diamag...
Text Solution
|
- Among the following element which shows shows only 'one' non-zero oxid...
Text Solution
|
- Which of the following is incorrect ?
Text Solution
|