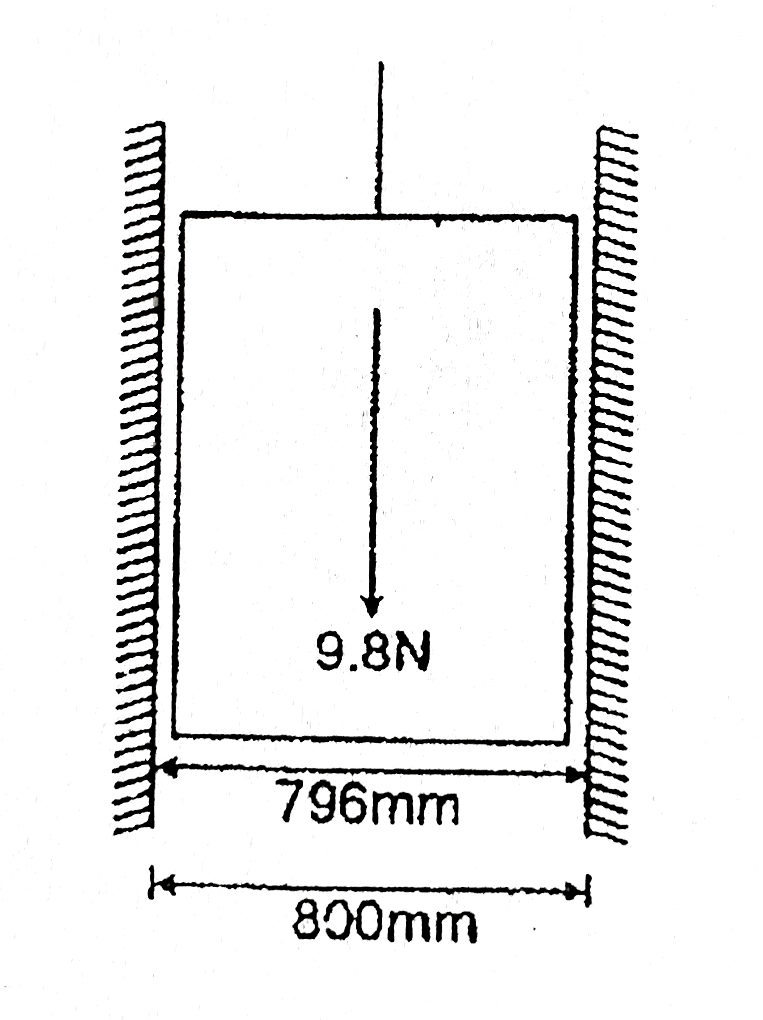Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELASTICITY AND VISCOCITY
RESONANCE|Exercise Exercise- 2 PART - III|11 VideosELASTICITY AND VISCOCITY
RESONANCE|Exercise Exercise- 3 PART - I|7 VideosELASTICITY AND VISCOCITY
RESONANCE|Exercise Exercise- 2 PART - I|11 VideosDAILY PRACTICE PROBLEMS
RESONANCE|Exercise dpp 92 illustration|2 VideosELECTROMAGNETIC INDUCTION
RESONANCE|Exercise Exercise|43 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-ELASTICITY AND VISCOCITY-Exercise- 2 PART - II
- A rod 1 m long is 10 cm^(2) in area for a portion of its length and 5 ...
Text Solution
|
- The cross-section of a bar is given by [1 + (x^(2))/(100)]cm^(2), wher...
Text Solution
|
- Two blocks A and B are connected to each other by a string and a sprin...
Text Solution
|
- A thin ring of radius R is made of a material of density rho is Young'...
Text Solution
|
- A uniform copper bar of density rho, length L, cross-sectional area S ...
Text Solution
|
- A piston of 796 mm diameter and 200 mm long works in a cylinder of 800...
Text Solution
|
- If the approximate viscosity of the oil for the following case is lamb...
Text Solution
|
- A circular disc of a diameter 'd' slowly rotated in a liquid of large ...
Text Solution
|