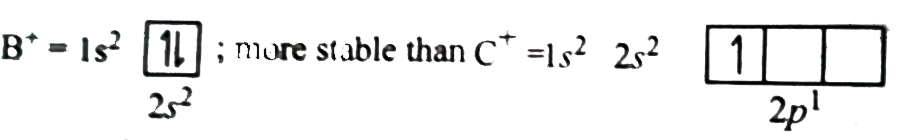Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC CLASSIFICATION AND PERIODIC PROPERTIES
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise OBJECTIVE_TYPE|1 VideosPERIODIC CLASSIFICATION AND PERIODIC PROPERTIES
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise COMPREHENSION_TYPE|3 VideosP BLOCK ELEMENTS
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise Topic 2|1 VideosQUALITATIVE ANALYSIS
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise Assertion and Reason|2 Videos
Similar Questions
Explore conceptually related problems
IIT-JEE PREVIOUS YEAR (CHEMISTRY)-PERIODIC CLASSIFICATION AND PERIODIC PROPERTIES-COMPREHENSION_TYPE
- Give reason for the following : a. The first ionisation enthalpy of ...
Text Solution
|
- Let's consider a hypothetical planet "pseudo Earth" which is similar t...
Text Solution
|
- Let's consider a hypothetical planet "pseudo Earth" which is similar t...
Text Solution
|
- Let's consider a hypothetical planet "pseudo Earth" which is similar t...
Text Solution
|