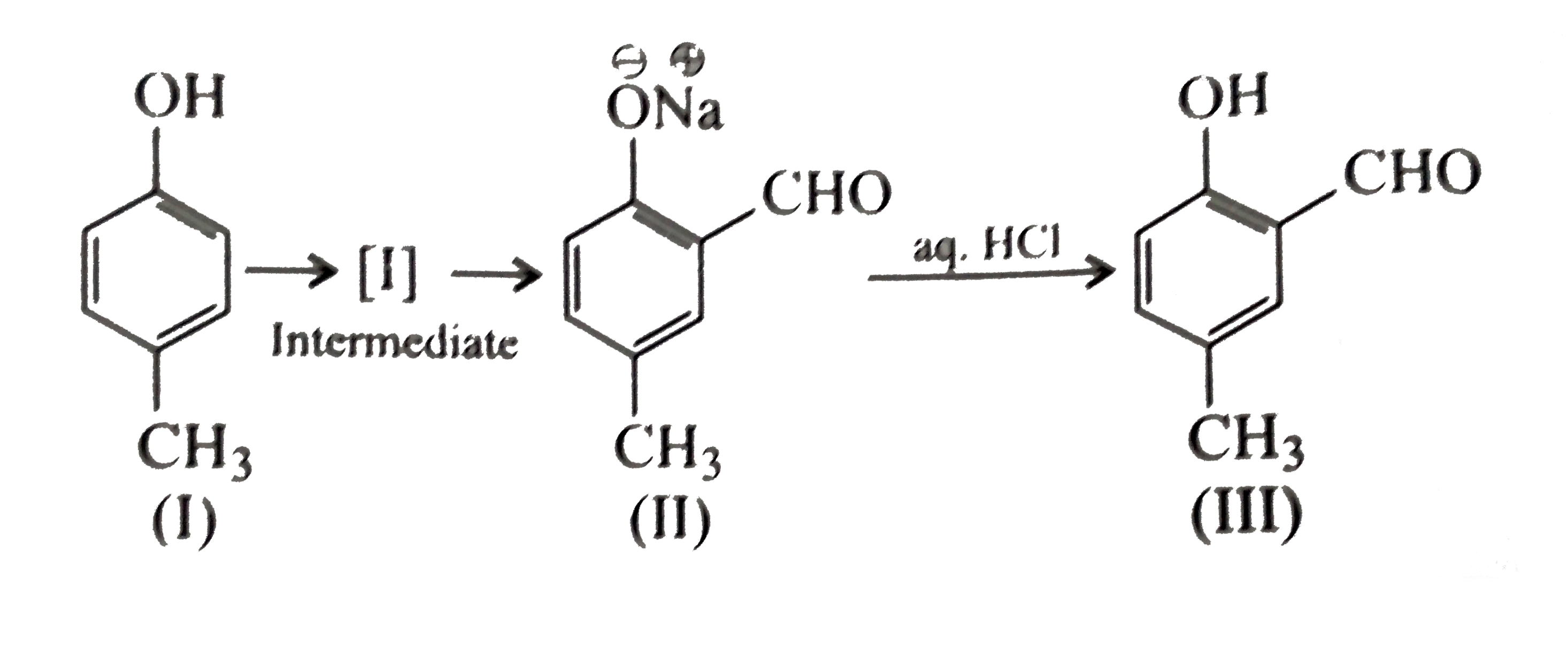
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
IIT-JEE PREVIOUS YEAR (CHEMISTRY)-ARYL HALIDE AND PHENOLS-JEE Main And Advanced
- Assertion: Aryl halides undergoes nucleophilic substitution with ease...
Text Solution
|
- Reimer-Tiemann reaction introduces an aldehyde group on to the aromati...
Text Solution
|
- Reimer-Tiemann reaction introduces an aldehyde group on to the aromati...
Text Solution
|
- Reimer-Tiemann reaction introduces an aldehyde group on to the aromati...
Text Solution
|
- The number of resonannce structure for N is
Text Solution
|
- Amongst the three isomers of the nitrophenol, the one that is least so...
Text Solution
|
- Phenol is acidic because of the resonance stabilisation of its conjuga...
Text Solution
|
- Formation of phenol from chlorobenzene is an example of …. Aromatic ...
Text Solution
|
- The acidity of phenol is due to the ….. Of its anion.
Text Solution
|
- Carry out the following conversation. Phenol to aspirin Benzoic ac...
Text Solution
|
- How would you synthesise 4-methoxyphenol from bromobenzene in not mo...
Text Solution
|
- What would be the major product in the following reaction?
Text Solution
|
- Explain briefly the formation of the products giving the structures ...
Text Solution
|
- Complete the following, giving the structures of the principal orga...
Text Solution
|
- How will you prepare m-bromoiodobenzene from benzene (in not more t...
Text Solution
|
- Explain the following in one or two sentence only: 'Phenol is an acid...
Text Solution
|
- Complete the following with appropriate structures:
Text Solution
|
- Compound A,C(7)H(8)O, is insoluble in water, dilute HCl, and aquenous ...
Text Solution
|
- Give reason in one or two sentences form the following: 'o-nitrophenol...
Text Solution
|
- Which of the following compounds undergoes nucleophilic substitutio...
Text Solution
|